Bovine Abortion
- Cosme Sánchez-Miguel
- Senior Research Officer, Cork Regional Veterinary Laboratory, Model Farm Road, Bishopstown, Cork, Ireland
Abortion in ruminants is a significant cause of economic loss. Laboratory diagnosis is central to managing and controlling outbreaks, limiting their spread and preventing zoonotic infections. While many pathogens can cause abortion in cattle, no single diagnostic test can be used to identify all aetiologies. Regional Veterinary Laboratories (RVLs) foetal investigations primarily focus on the most likely aetiologies and those with zoonotic potential. Brucellosis, an important disease, has been eradicated in Ireland following a successful statutory program; however, continuous surveillance remains crucial for both public and animal health considerations.
A threshold of 5 per cent foetal mortality rate of is recommended when deciding whether to instigate an investigation, although in some instances, a cluster of cases in quick succession may be more critical in deciding to submit aborted material to the laboratory. The aetiology of bovine abortion is broad and diagnostic success rate is low, however, adequate sampling, appropriate laboratory testing, clinical history, vaccination programme and epidemiological information increase chances of reaching an aetiologic diagnosis.
Aborted foetus, placenta and maternal serum constitute the minimum sampling requirements for an abortion investigation. The inclusion of placenta is critical for diagnosis of some mycotic and bacterial abortions where placenta is the primary tissue affected. Submission of blood samples from aborting cows can provide valuable information by either excluding some organisms, i.e. Neospora caninum, or reinforcing diagnosis of other agents, as is the case in Salmonella Dublin abortions.
In 2018, 1914 bovine foetuses, stillbirths and foetal material (placenta, foetal organs, abomasal contents, etc.) were tested for Brucellosis and routine foetal cultures in RVLs. This section presents some of the most common aetiologies diagnosed in RVLs. Bacterial, fungal and protozoal agents are the most frequent abortifacients detected; they can be divided into primary and secondary pathogens. Primary pathogens can cross an intact placenta and cause placentitis and fetopathy. Secondary pathogens are opportunistic organisms that count on maternal immunosuppression or placental damage to cause abortion .
In routine RVL foetal culture workflows, most bacteria associated with abortion in cattle can be isolated by aerobic culture from abomasal contents, placenta or foetal organs. Anaerobic culture is not usually carried out in this workflow, therefore, anaerobic bacteria may be underreported as abortifacients. Similarly, organisms that required specific media culture, e.g. Mycoplasma spp, Ureaplasma or Chlamydophila spp, may also be underreported.
Primary Pathogens
Agents such as Brucella abortus, Salmonella Dublin, Leptospira harjo, Listeria monocytogenes, Aspergillus fumigatus, Neospora caninum, BVDv, BHV-1, etc., are capable of crossing intact placentas causing placentitis, fetopathy and/or luteal regression; they are classified as primary abortifacients.
Some abortifacients are zoonotic and can pose a serious threat to the health of veterinary practitioners and farmers. It is advisable to always take precautions when handling foetuses or aborted material.
Salmonella Dublin abortion
Salmonella abortions in Ireland are predominantly associated with Salmonella Dublin serotype. In 2018, 4.21 per cent of bovine abortions were attributed to Salmonella Dublin (Table 6.1. This type of abortion usually occurs in the second half of pregnancy with bacterial translocation from the intestine to the placenta. Typically, aborted foetuses are autolysed, occasionally emphysematous, and smell of rotten eggs due to production of hydrogen sulphide. A diagnosis of Salmonella Dublin can also be reached with maternal serology. In non-vaccinated aborting cows a single blood sample can be up to 85 per cent accurate in predicting a S. Dublin foetal culture positive result (Sánchez-Miguel et al. 2018).
Table 6.1: Number of Salmonella Dublin isolates in foetal material in 2018 (n= 1970 ).
|
Result
|
No. of Cases
|
Percentage
|
|
Negative
|
1887
|
95.8
|
|
Positive
|
83
|
4.2
|
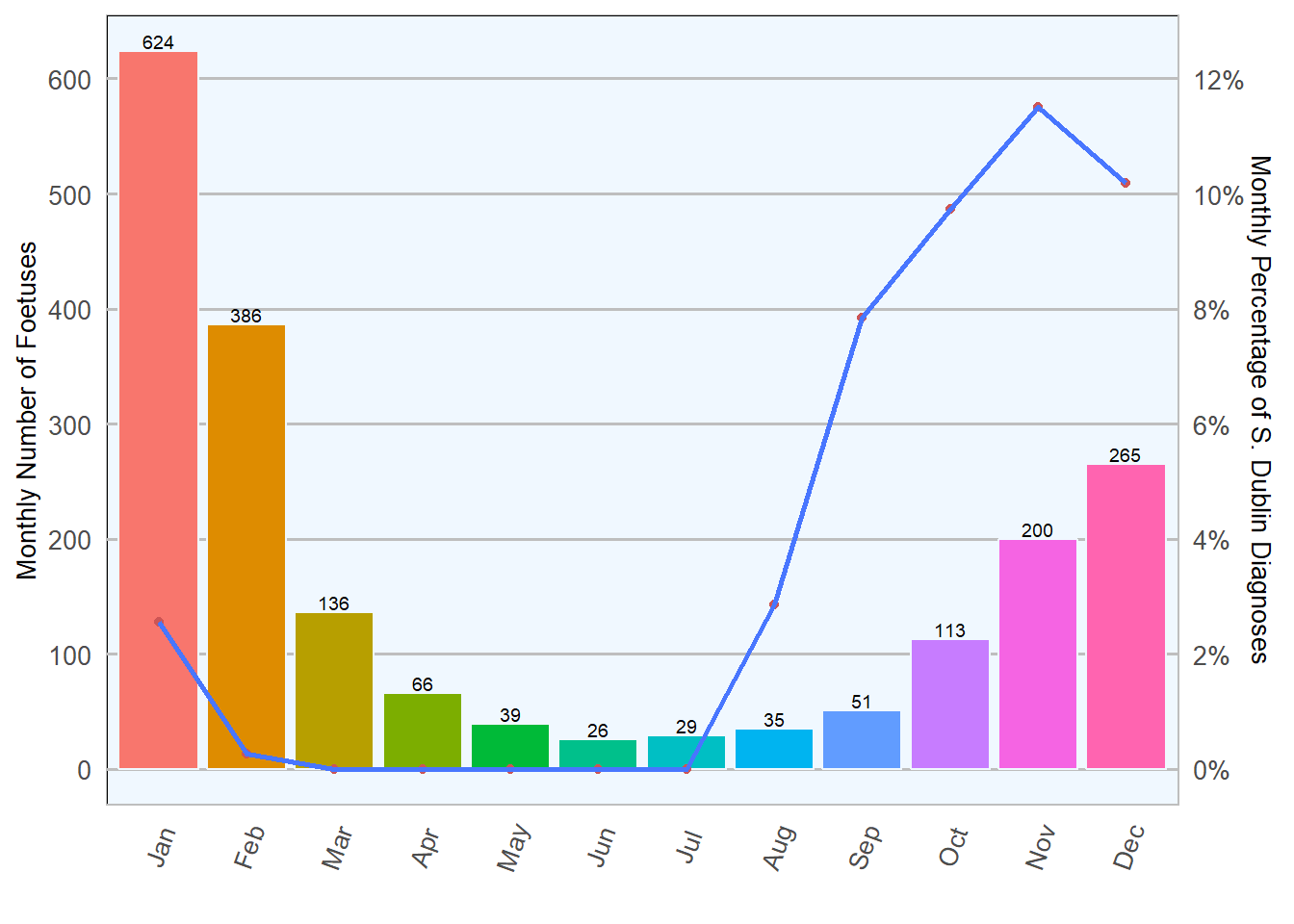
Salmonella Dublin abortions have a well documented seasonal distribution in Ireland characterised by a steady increase towards October/November, as shown in Table 6.2 and Figure 6.2; this seasonal distribution emphasises the importance of choosing the right time to vaccinate for Salmonella Dublin.
Table 6.2: Monthly count and percentage of Salmonella culture results in foetal material during 2018 (n= 1970 ).
|
Month
|
Total
|
Positive
|
Percentage
|
|
Jan
|
624
|
16
|
3
|
|
Feb
|
386
|
1
|
0
|
|
Mar
|
136
|
0
|
0
|
|
Apr
|
66
|
0
|
0
|
|
May
|
39
|
0
|
0
|
|
Jun
|
26
|
0
|
0
|
|
Jul
|
29
|
0
|
0
|
|
Aug
|
35
|
1
|
3
|
|
Sep
|
51
|
4
|
8
|
|
Oct
|
113
|
11
|
10
|
|
Nov
|
200
|
23
|
12
|
|
Dec
|
265
|
27
|
10
|
Nine Salmonella spp. serotypes other than S. Dublin were also isolated in foetuses, eight were Salmonella typhimurium and one Salmonella Indiana 6.4.
Listerial abortion
Listeria monocytogenes and possibly L. ivanovii may cause sporadic abortions in all stages of pregnancy. Listeria spp. are widespread in the environment; clinical disease is associated with ingestion of poorly fermented silage. Following ingestion, Listeria monocytogenes proliferates firstly in placenta, then in foetal liver causing septicaemia and, lastly, death.
The proportion of diagnosed abortions attributed to L. monocytogenes infection is usually is low, amounting to 42 (2.19 per cent) of the total abortions during 2018 (Table 6.3. Most listerial abortions have a sporadic occurrence and are rarely associated with listerial encephalitis. A markedly autolysed foetus is usually aborted in the third trimester.
Table 6.3: Frequency of detection of other primary abortion pathogens in foetal culture during 2018 (n= 1970 )
|
Organism
|
No. of cases
|
Percentage
|
|
Trueperella pyogenes
|
131
|
6.8
|
|
Bacillus licheniformis
|
101
|
5.3
|
|
Listeria moncytogenes
|
42
|
2.2
|
|
Aspergillus spp
|
12
|
0.6
|
Leptospiral abortion
Leptospira hardjo has adapted to cattle, which serve as maintenance host. Leptospira spp. is labile and difficult to culture, hence diagnosis normally relies on detection of antibody titres by foetal serology or, occasionally, on Fluorescent Antibody Test (FAT) on foetal kidney smears using multivalent antisera or PCR for pathogenic Leptospira spp.. Leptospirosis is likely to be underdiagnosed as cause of abortion in cattle due to poor diagnostic tests available at present. This may explain the variability in percentages of diagnosed cases from year to year and laboratory to laboratory. Abortion is frequently the only clinical sign observed in a herd, except in lactating cattle where signs of acute leptospirosis may include agalactia, mastitis, fever, haemolytic anaemia, haemoglobinuria and icterus.
Minor Primary Abortifacients (sporadic abortions)
Some bacteria can cause maternal bacteraemia, reach the gravid uterus and foetus and progress to causing sporadic abortion. Amongst them, Truperella pyogenes, with 131 (6.84 per cent) and Bacillus licheniformis with 101 cases (5.28 per cent) are listed as the most common agents of sporadic abotion.
Protoozoal abortion
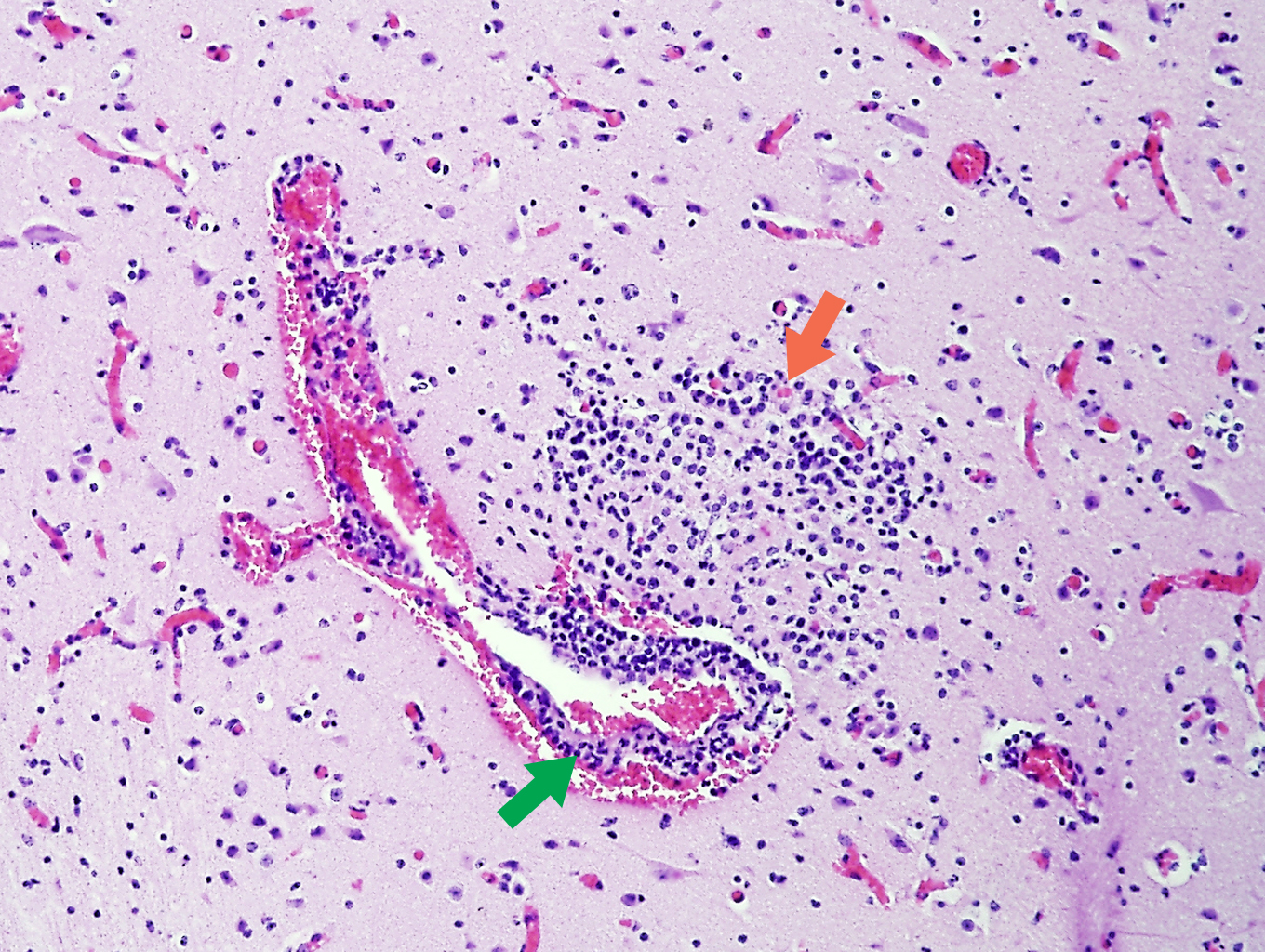
Since its identification in the 80’s, neosporosis, caused by the protozoan Neospora caninum, has emerged as one of the most common infectious causes of abortion in cattle worldwide. Acutely infected dogs shed N. caninum oocysts in faeces contaminating the environment. Cattle may become infected by ingesting oocysts (from infected aborted material or environment) or by acquiring the parasite in utero. The parasite invades and multiplies within placental cells causing impairment of oxygen and nutrient transfer from mother to foetus, leading to foetal death. N. caninum may also reach foetal organs causing a non-suppurative inflammatory reaction; foetal brain, followed by myocardium, are the preferred sites to detect characteristic lesions (Figure 6.3.
In 2018, N. caninum was detected in 81 foetuses, either by foetal serology, histological examination or by both methods. This figure represents a similar proportion of cases in 2018 compared to 2016, 4 per cent and 3.5 per cent of the total number of foetuses respectively; however, it is essential to bear in mind that not every submitted foetus is tested for N. caninum.
Most N. caninum abortions occur in mid to late gestation, but not all cows that are infected with N. caninum will abort. Nonetheless, infected cows are more likely to abort than uninfected. N. caninum abortions are more frequently seen in heifers or recently infected cows. This type of abortion follows different patterns that are dependent on level of exposure to parasite and predominant route of transmission within the individual herd. These patterns are:
- Epidemic abortions (abortion storms): due to primary infection of naive cows that are exposed to a single source of infection such as ingestion of aborted membranes, feed or water contaminated with N. caninum oocysts.
- Endemic abortions: chronic abortion episodes spanning several years and found within infected family lines as a result of recurrent transplacental (vertical) transmission.
- Sporadic abortions: occasional occurrence of abortions within a herd
In RVLs, diagnosis of bovine neosporosis at post-mortem is based on presence of lesions consistent with protozoal damage in infected tissues (brain, myocardium and placenta) and detection of specific antibodies in the dam or foetal blood or fluids. Detection of N. caninum by PCR or immunohistochemistry in tissues is not undertaken in routine foetal submissions and is only carried out occasionally in herd investigations. Diagnostic of N. caninum abortions poses a two-fold challenge: tissue lesions, though very distinctive (necrotic foci and mononuclear cell infiltrates) are only suggestive of protozoal abortion and foetal serology depends on quality of the sample (absence of autolysis) and age of the foetus (mature enough to have produced antibodies). In addition to that, a serology positive N. caninum test result should be viewed with caution as calves are not always adversely affected by the protozoa and abortion could have been caused by a different abortifacient agent.
Control options for Neospora infection are based on biosecurity, identification of infected animals and appropriate management decisions. An integrated control programme should include measures aimed at minimising chances of horizontal (ingestion of infective oocysts) and vertical (from mother to foetus) transmission thus interrupting the parasite life-cycle.
Prevent Neospora transmission by enhancing biosecurity
Dispose of aborted materials (foetuses and placenta) promptly and safely, as tissues infected by Neospora and other abortificient agents pose a high risk of infection.
Prevent dogs from having access to cattle areas, especially calving areas.
Prevent dogs from having access to cattle feed, pastures, fields for production of cattle forage and water sources.
- Control rodents on the farm. Rodents may act as intermediate hosts for Neospora and they may pose a risk if ingested by dogs.
Table 6.4: Combined frequency of detection of selected abortion agents on routine foetal culture.
|
Organism
|
No of Cases
|
|
Coliforms
|
333
|
|
Streptococcus spp
|
61
|
|
Bacillus spp
|
11
|
|
Yeasts and Fungi
|
10
|
|
Salmonella spp (other than S. dublin)
|
9
|
|
Staph. spp
|
9
|
|
Listeria spp
|
7
|
|
Mannheimia haemolytica
|
6
|
|
Pseudomonas spp
|
5
|
|
Pasteurella multocida
|
2
|
|
Histophilus somnus
|
1
|
|
Yersinia pseudotuberculosis
|
1
|
Secondary Pathogens
These organisms form a diverse group of bacteria associated with opportunistic infections of placenta and foetus; they are incapable of transplacental infection unless there is a damage to the placenta or dam is immunocompromised. Since their presence is widespread in the environment, they can potentially cause maternal bacteraemia, reach the gravid uterus and trigger an opportunistic abortion. Table 6.4 summarises the number of cases in 2018, amongst them Streptococcus spp. (61 cases isolated), Bacillus spp (61), Staphylococcus spp. (9), Mannheimia haemolytica (6), Pseudomonas spp. (5), Pasteurella multocida (2), Histophilus somnus (1), Yersinia pseudotuberculosis (1).
Their presence in tissues of aborted foetuses should not be considered as definitive evidence of cause of abortion. For secondary pathogens to be the regarded as cause of abortion, they must be isolated from foetal material, have produced representative lesions and primary pathogens must have been excluded. Secondary pathogens usually cause sporadic abortions; multiple abortions can be a consequence of maternal health issues that facilitate haematogenous infections.
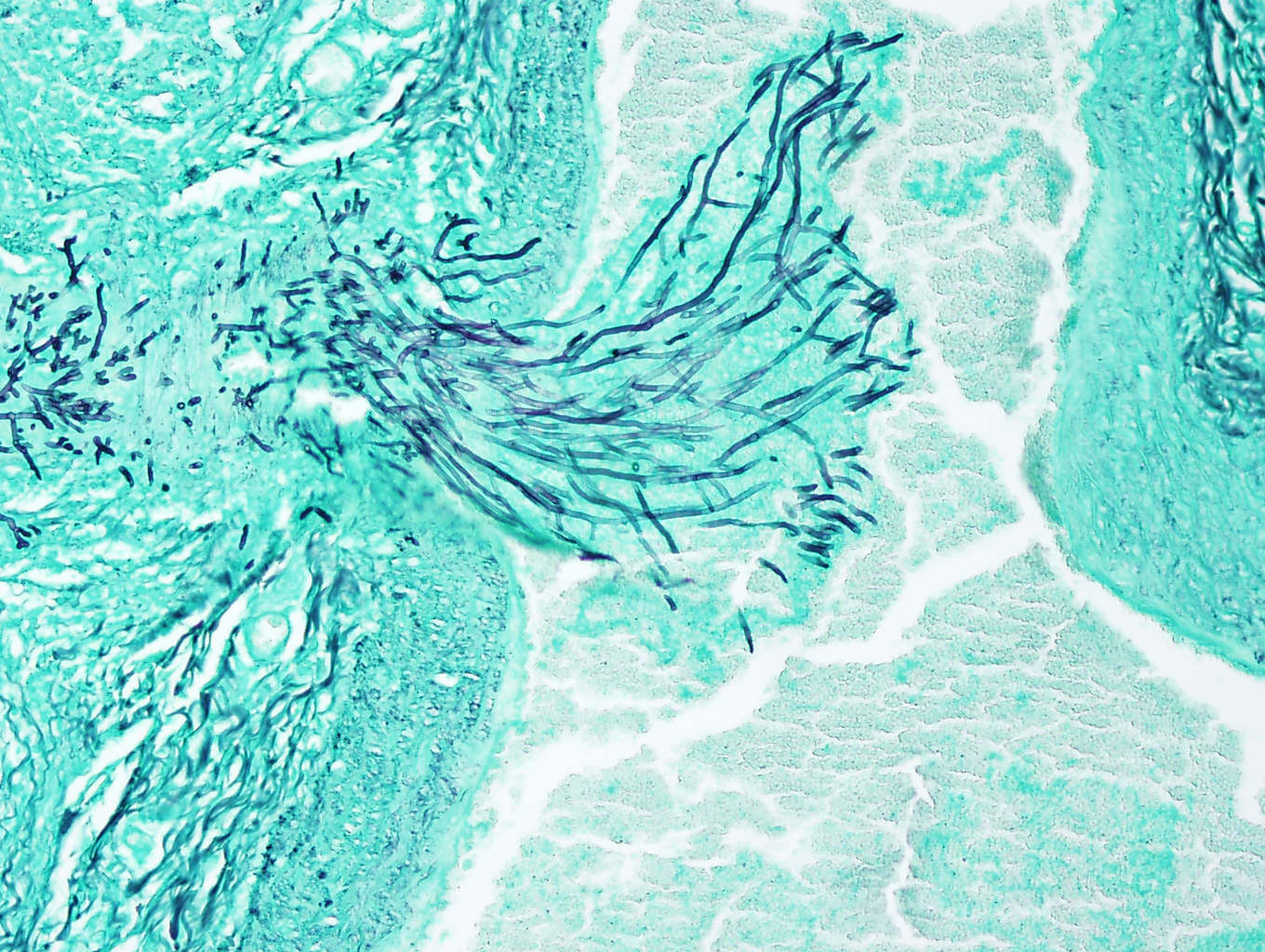
Mycotic abortions
Mycotic abortions usually occur in the third trimester of pregnancy. Aspergillus spp. (Figure 6.4 and Mucor spp. are the most common organisms isolated (10 cases in 2018). Clinical signs in dams, apart from placental retention, are infrequently observed. Diagnosis of fungal abortion is based on demonstration of fungi and presence of consistent gross and histopathological lesions. Grossly visible placental lesions include a leathery, diffusely thickened intercotyledonary membrane with necrotic haemorrhagic infarcts in cotyledons. Foetal lesions may be absent and autolysis minimal. Occasionally, locally extensive circular skin lesions may be present on foetuses. Microscopically, there is a severe suppurative placental vasculitis with intralesional fungi . Inflammatory lesions associated with fungal invasion may be present in foetal respiratory and digestive systems. Direct identification of fungi using a potassium hydroxide wet-mount examination of lesion scrapings may facilitate diagnosis.