
Section 2 Johne’s Disease
- Aideen Kennedy
- Research Officer, Kilkenny Regional Veterinary Laboratory, DAFM, Leggatsrath, Hebron Road, Kilkenny, Ireland
Johne’s disease (JD) is a chronic granulomatous enteritis of ruminants caused by Mycobacterium avium subspecies paratuberculosis (MAP). Clinical JD is characterised by diarrhoea and progressive cachexia and ultimately results in death.
In total, seventy seven positive MAP faecal cultures, from 53 different herds, were recorded in 2018 (Figure 2.2). Ten herds had more than one positive culture, with seven being the highest number of MAP faecal positive cultures in one single farm. Over 90 per cent of positive animals were female. A mixture of dairy and beef breeds recorded positive results. However, almost 30 per cent of positive samples were from Holstein Friesians (Table 2.1).
| Breed | Female | Male | Total |
|---|---|---|---|
| Holstein Friesian | 22 (31.0) | 1 (16.7) | 23 (29.9) |
| Limousin | 12 (16.9) | 1 (16.7) | 13 (16.9) |
| Charolais | 9 (12.7) | 0 (0.0) | 9 (11.7) |
| Friesian | 8 (11.3) | 0 (0.0) | 8 (10.4) |
| Jersey | 8 (11.3) | 0 (0.0) | 8 (10.4) |
| Aberdeen Angus | 4 (5.6) | 3 (50.0) | 7 (9.1) |
| Belgian Blue | 3 (4.2) | 0 (0.0) | 3 (3.9) |
| Shorthorn | 1 (1.4) | 1 (16.7) | 2 (2.6) |
| Blonde D’Aquitaine | 1 (1.4) | 0 (0.0) | 1 (1.3) |
| Hereford | 1 (1.4) | 0 (0.0) | 1 (1.3) |
| Norwegian Red | 1 (1.4) | 0 (0.0) | 1 (1.3) |
| Partanaise Cross | 1 (1.4) | 0 (0.0) | 1 (1.3) |
Latency is a common feature of mycobacterial diseases, animals can remain sub-clinically infected without showing any clinical signs of the disease for many years. Clinical disease is reported to occur most frequently in cattle aged 2–5 years. In line with this, based on a number of assumptions, it is estimated that 50 per cent of animals that tested positive in 2018 were displaying symptoms of JD by four years of age (Figure 2.3). At time of analysis, all male animals that had a positive result in 2018 were no longer alive and only 24 per cent of positive female animals were still alive (Figure 2.4).
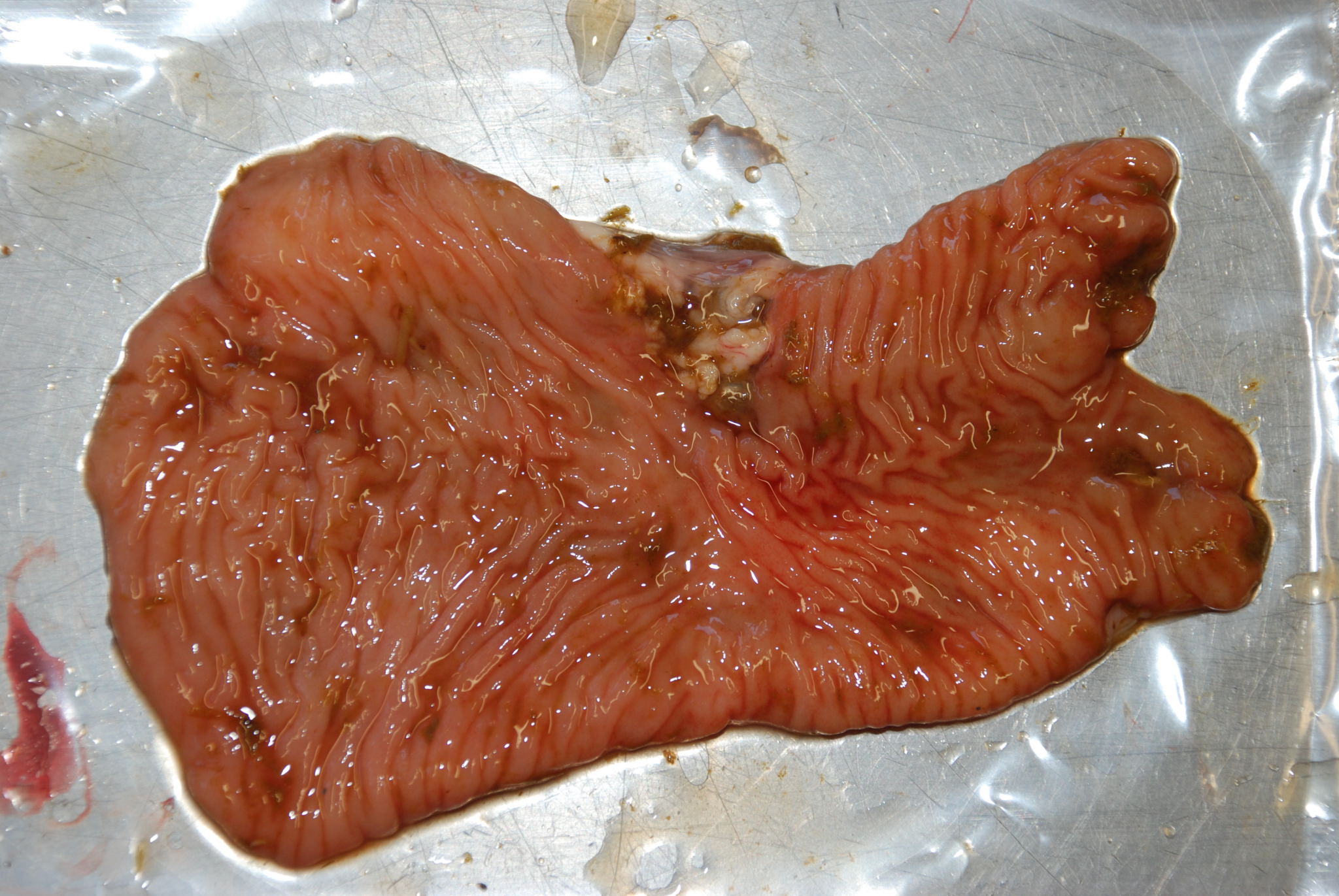
Figure 2.1: Thickened and wrinkled intestinal mucosa (granulomatous enteritis) in the ileum of a cow with Johne’s disease (Mycobacterium avium ssp. paratuberculosis). Photo: Cosme Sánchez-Miguel.
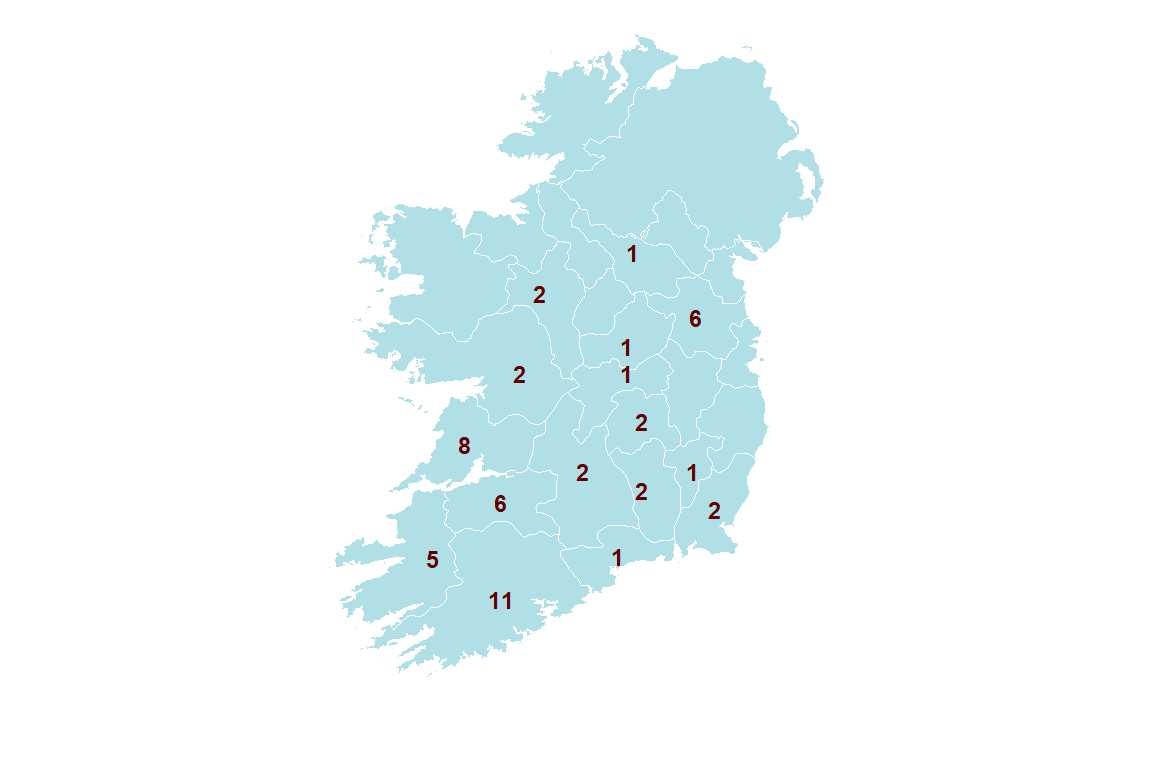
Figure 2.2: Number of herds by county with at least one animal diagnosed with JD by faecal mycobacterial culture in 2018.
2.1 JD transmission
In many herds, initial introduction of MAP usually occurs as result of acquiring an infected but clinically normal animal. In 2018, a number of animals that subsequently recorded MAP faecal culture positive results underwent multiple herd movements throughout their lifetime, potentially allowing spread of the disease. Five was the greatest number of herd movements recorded by a positive animal, excluding movements to factory or knackery (Table 2.2).
| Minimun | Median | Maximun |
|---|---|---|
| 0 | 1 | 5 |
Once MAP is introduced to a herd, infection with MAP is understood to occur, primarily, as a calf. Animals younger than six months are believed to be the most susceptible. Neonates are considered to be at highest risk of acquiring MAP infection due to increased permeability of intestines during the first 24 hours of life and an immature immune system. Older animals are believed to be less susceptible; however, infection can still occur (Windsor and Whittington 2010).
Severity and rate of JD progression in individual animals are dependent on MAP exposure dose and age at time of infection. Infection usually occurs via the faecal-oral route, although in-utero transmission can also occur. Exposure of calves to adult faeces is the most important risk factor in MAP transmission (Doré et al. 2012). Faecal-oral transmission is facilitated by faecal contamination of feedstuff and calf’s environment, with highest environmental risk factors for neonatal infection being faecal contamination of udders and calving pens. Colostrum and milk from infected cows can also contain quantities of MAP capable of infecting calves. Feeding pooled colostrum or milk from multiple cows of unknown MAP status is considered to increase risk of infection within a herd.
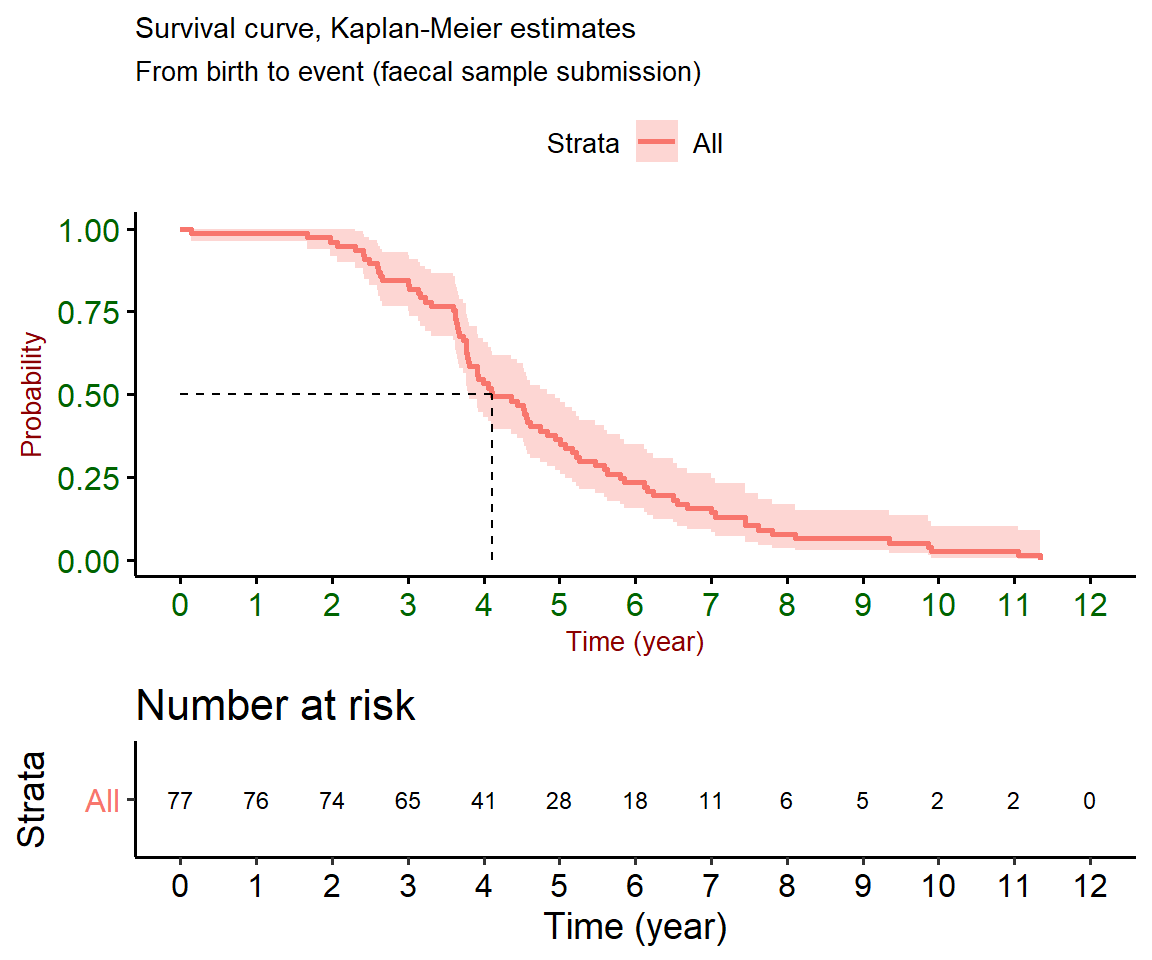
Figure 2.3: Survival curves measure how much time elapsed before a certain event occurred. In this case, the event is represented by submission of a faecal sample to an RVL. An assumption is made that faecal samples are submitted soon after the animal displays diarrheoa unresponsive to treatment. 50 % of animals may have displayed symptoms consistent with the disease by four years of age. The graph on the bottom represents number of animals at risk of developing symptoms over time.
Figure 2.4: Status of animals diagnosed in 2018 with Johne’s disease as per the 25 of April, 2019
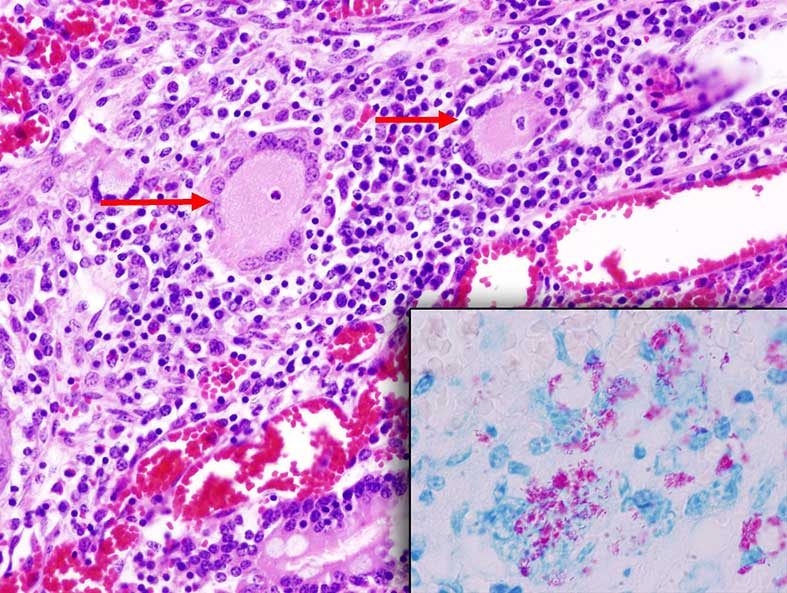
Figure 2.5: Microphotography of Langhan’s-type giant cells (arrows) occasionally observed in tuberculoid granulomas seen in the lamina propia of the small intestine in animals with Johne’s disease. Inset: Ziehl-Neelsen stained section showing acid-fast (Mycobacterium avium ssp. paratuberculosis bacilli). Photo: Cosme Sánchez-Miguel.
2.2 JD Diagnostics
As treatment of MAP is generally regarded as ineffective, diagnostic testing is often used to direct subsequent management decisions (e.g. calf in separate area, cull, etc.) and allow preventative management messures of non-infected herd mates. As MAP is a slow growing bacterium, infection can remain latent for many years making diagnosis difficult. Diagnostic tests currently in use involve either identification of MAP itself (culture), identification of MAP genetic elements (PCR), or detection of the immune response MAP infection elicits (ELISA) (Behr and Collins 2010).
Faecal culture is generally taken as the reference test for MAP. An advantage of culture is that detection of MAP in faecal samples confirms presence of viable MAP in an animal. Due to absent or intermittent shedding of bacteria early in the disease process, sensitivity of culture can be low. Specificity, however, is almost 100 per cent. Due to the fastidious nature of MAP, culture takes a number of weeks. Polymerase Chain Reaction (PCR) is another faecal based test used to detect DNA of MAP, it offers a rapid method of detecting MAP status.
Enzyme Linked Immune Sorbent Assay (ELISA) examines the host’s immune response to MAP and is extensively used for routine diagnosis. ELISA is favoured as a screening test due to its relatively low cost, compared to faecal culture or PCR. ELISAs also provide faster results when compared to culture methods. ELISA relies on identifying serum antibodies to a particular antigen as an indicator of infection. It is important to note that a positive ELISA reaction is NOT confirmation of JD. The specificity of MAP ELISA tests can be influenced by tuberculin testing and by exposure to non-MAP environmental mycobacteria (giving rise to false positive results). The sensitivity of MAP ELISA tests is influenced by stage of infection, high in animals with clinical disease but low in infected animals that are shedding few MAP organisms (where false negative results may arise).
2.3 Post mortem examination
On post mortem, gross and microscopic lesions associated with JD are primarily confined to the intestine and mesenteric and ileo-caecal lymph nodes. Gross lesions are characterised by thickening and corrugation of intestinal mucosa, most prominent in distal ileum and ileo-caecal valve. Histological lesions associated with JD can vary widely; villi are frequently fused and mucosa is invariably thickened, infiltration of macrophages -including giant cells- is commonly identified in the submucosa and acid fast bacilli are commonly present. JD cannot be diagnosed solely on post mortem, diagnosis needs to be confirmed by faecal culture and/or histology (intestine/lymph nodes).
2.4 Control Programme
A voluntary national JD control programme is on-going in Ireland under the guidance of Animal Health Ireland. The aim is to provide pathways for test-negative and test-positive herds to demonstrate progress towards an improved herd assurance for JD. Primary aspects of this programme involve identification of potentially infected animals via either milk or blood ELISA testing, confirmation relying on faecal based testing. Highlighting on farm management practices using veterinary risk assessment and management plans (VRAMP) is commonly used as a tool in a number of control programmes, including AHI’s JD control programme. VRAMP is a combined work between a farmer and a trained local vet familiar with his/her farm which facilitates identifying specific high risk management practices occurring in such farm that may facilitate spread of JD. Repeat visits allow monitoring of successful implementation of management changes.
2.4.1 Acknowledgement
Dr Kevin Kenny (TB Section, DAFM) for providing the JD dataset and Alma Wilson (Cork RVL, DAFM) for sorting out this data for analysis.
References
Windsor, Peter A., and Richard J. Whittington. 2010. “Evidence for Age Susceptibility of Cattle to Johne’s Disease.” The Veterinary Journal 184 (1): 37–44. doi:https://doi.org/10.1016/j.tvjl.2009.01.007.
Doré, E., J. Paré, G. Côté, S. Buczinski, O. Labrecque, J.P. Roy, and G. Fecteau. 2012. “Risk Factors Associated with Transmission of Mycobacterium Avium Subsp. Paratuberculosis to Calves Within Dairy Herd: A Systematic Review.” Journal of Veterinary Internal Medicine 26 (1): 32–45. doi:10.1111/j.1939-1676.2011.00854.x.
Behr, Marcel A, and Desmond M Collins. 2010. Paratuberculosis: Organism, Disease, Control. CABI.
A cooperative effort between the VLS and the SAT Section of the DAFM