
Section 13 Ovine Parasitic Diseases
- Jimmy Wiseman
- Research Officer, Central Veterinary Research Laboratory, Backweston Campus, Young’s Cross, Celbridge, Co. Kildare. Ireland.
Parasitic disease is consistently one of the most frequent post-mortem diagnosis in Regional Veterinary Laboratories (RVLs). In addition, faecal samples are submitted for clinical diagnosis; the majority of these samples come from herds or flocks with animals showing clinical signs of diarrhoea and/or weight loss. Fasciola hepatica (Fasciolosis),Trichostrongylidae (Parasitic Gastro-Enteritis) and Dictocaulus viviparus (Parasitic Pneumonia) are the most important organisms responsible for parasitic disease in cattle and sheep.
13.1 Agents of Parasitic Gastroenteritis
Faecal samples from young lambs are typically analysed for presence of trichostrongyle and nematodirus eggs, and coccidial and cryptosporidial oocysts. In older lambs (> 12 weeks) cryptosporidial oocysts is substituted for fluke eggs. Results generally specify whether liver and/or rumen fluke eggs are present but, in cases where a significant rumen fluke issue is suspected, testing of duodenal contents may be preferable.
The following report outlines trends in the results of faecal tests carried out at RVLs in 2018 and provides some interesting material for consideration. It is important to remember, however, that there can be a poor correlation between severe infestations and faecal egg counts. Extensive disease, and even death, can occur in the pre-patent period (time between larval infestation and appearance of eggs in the faeces) and factors other than parasitic burden may influence the number of eggs parasites produce.
For this reason, particularly where there have been unexpected deaths, additional submission of fresh carcases for post-mortem examination should always be considered.
13.2 Trichostrongylidae
In general, life cycles of the gastrointestinal nematodes (gut worms) are similar. Species of particular importance include Teladorsagia circumcincta (formerly Ostertagia circumcincta), Trichostrongylus axei and Haemonchus contortus which dwell in the abomasum, and Trichostrongylus colubriformis and Cooperia curticei which inhabit the small intestine.
These parasites are unable to multiply within sheep and their life cycle is direct (without need of an intermediate host). Pre-patent period is 16–21 days, depending on particular species and various environmental and host factors (Abbott K.A. 2012).
Traditionally, infestations would have been associated with storing lambs at autumn. However, in recent years problems have increasingly been seen in growing lambs from mid-summer onwards.
All the aforementioned species produce eggs of typical trichostrongyle appearance and are undistinguishable at this stage, consequently, they are analysed together.
In RVLs, Trichostrongyle egg counts tend to be substantially higher in ovine faecal samples than in bovine faecal samples (DAFM 2016). There are many potential reasons for this though a greater focus on national ovine parasite control may be advisable.
| Result | No. of samples | Percentage |
|---|---|---|
| Negative | 642 | 38 |
| Low (50-500 epg) | 499 | 30 |
| High (>1200 epg) | 301 | 18 |
| Medium (500-1200 epg) | 231 | 14 |
Of 1673 samples analysed in 2018 (Table 13.1) just under a third (32 per cent) were suggestive of significant (medium or high) trichostrongyle burdens. This suggests that, whilst the majority of animals appear to be bearing a “manageable” parasite load, there are proportionally still too many with potentially damaging levels of infestation, an increase of 5 per cent comparing 2018 RVL figures with 2016 All-Ireland ADSR [DAFM (2016)}. In particular, the fact that there were more samples falling into the high burden category (18 per cent) than the medium one (14 per cent) is interesting as this does not reflect typical disease pattern. This may be suggestive of either issues with treatment (the possibility of anthelmintic resistance is always a concern) or else, perhaps, such results may indicate a bias towards testing only the most severely affected animals within the population, running the risk of overlooking those with less overt disease.
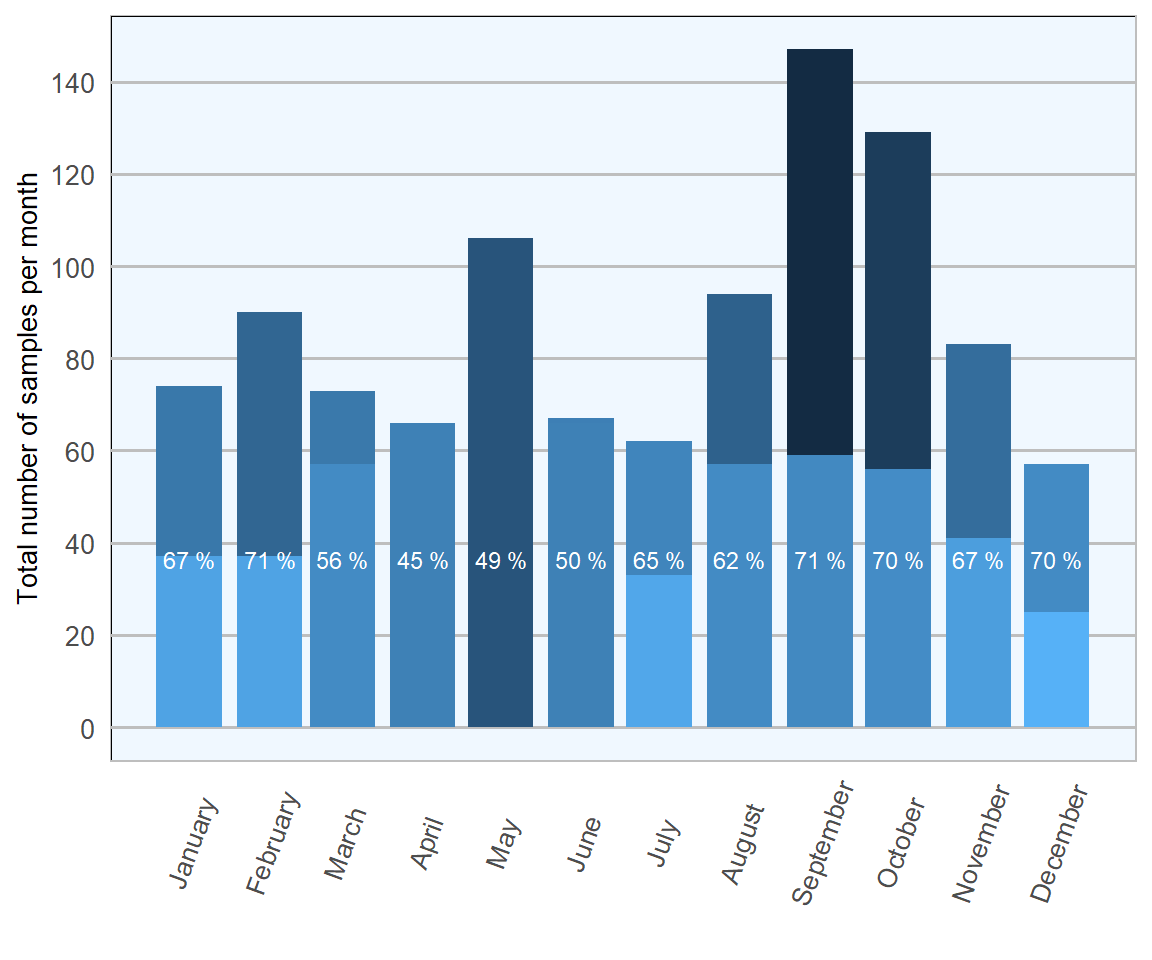
Figure 13.1: Stacked count of ovine faecal samples (all ages) tested per month for Trichostrongylidae during 2018. The percentage in each bar represents positive samples (n= 1673 ).
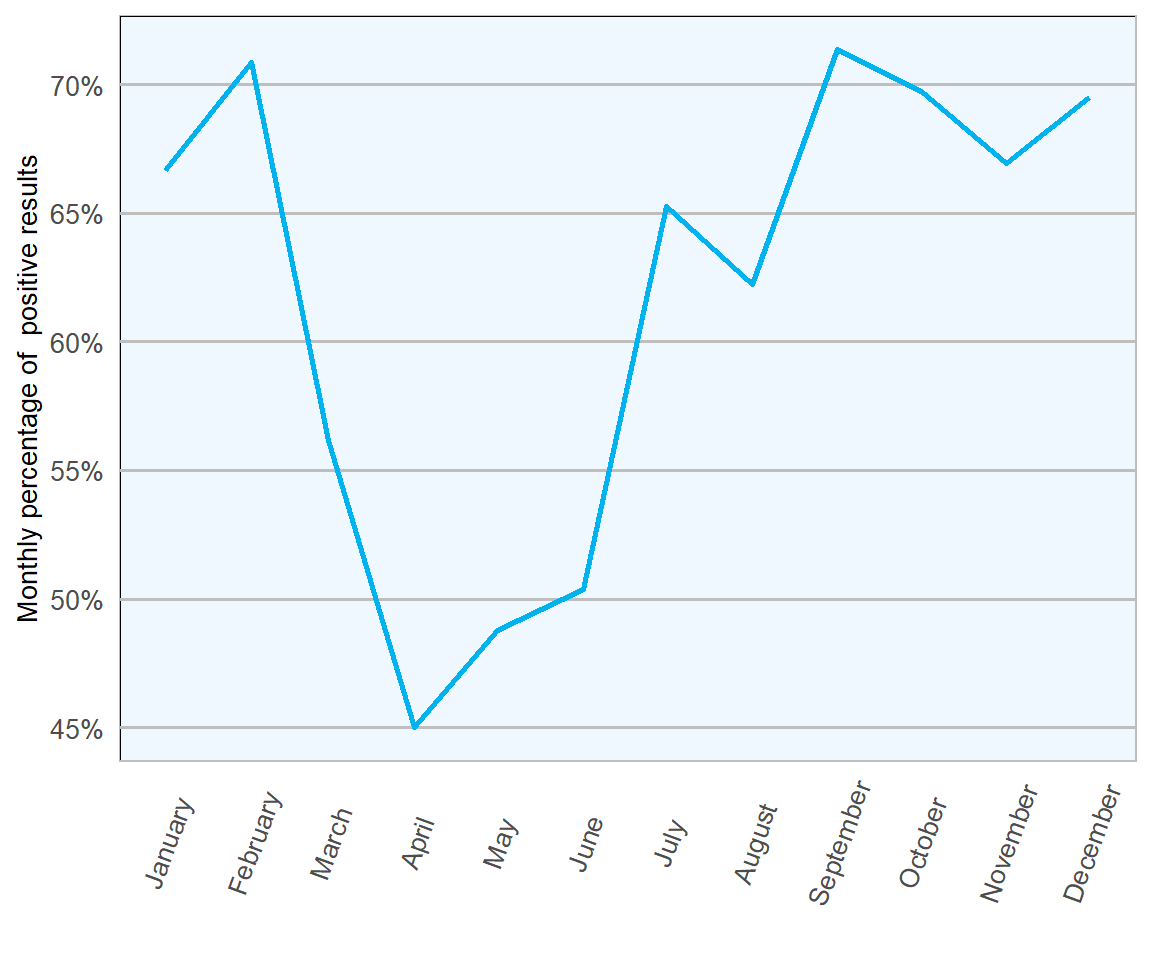
Figure 13.2: Percentage of positive ovine faecal samples for Trichostrongylidae eggs in 2018 (n= 1673 ).
The highest proportion of positive samples were examined in February and September (both 71 per cent), indicating peaks at either end of the grazing season (Figures 13.1 and 13.2). The immunomodulatory effects of parturition are known to result in increased faecal parasitic shedding, which may reflect the first peak. The second increase might indicate build-up of parasite numbers in pasture as the season progresses. Samples were least likely to be positive between April and June, in keeping with heavy parasitic burdens taking a while to develop in growing lambs, particularly prior to weaning.
Sample numbers examined typically averaged at about 120 per month, with peaks of >200 examined in both May and September, and lesser numbers (<100) in both July and December. A greater number of sample submissions in Autumn would be expected, as this would typically be the time of year when most problems are seen, but reasons for the peak in May are a little less obvious.
13.3 Nematodirus
Nematodirus battus is another small intestinal nematode of importance in the Trichostrongyloidea superfamily, in this report it is considered separately due to differences in life cycle and identification.
For all Nematodirus species, development as far as L3 (the infective larval stage) occurs within the eggs after they are deposited on pasture, this development takes several months. In the spring, the synchronised mass hatch of overwintered infective larvae is usually triggered by a sudden increase in environmental temperature following a chilled period, a temperature differential greater than is believed to be required for this phenomenon to occur (McMahon et al. 2017).
As a result, many farmers have traditionally limited the impact of this disease by rotating grazing on an annual basis and by managing their stock carefully during the period of highest risk in spring. Although it has been shown that up to 70 per cent of eggs may be capable of hatching without being first stimulated by chill temperatures (Dijk and Morgan 2008) and, in recent years, patterns of disease appear to be changing, with many farms reporting a second smaller peak in clinical cases towards the end of the grazing season.
Nematodirosis typically affects growing lambs in their first season, usually at 6–12 weeks of age. Thankfully, immunity develops quickly and clinical disease is not an issue in adult stock.
Nematodirus eggs can be easily differentiated from typical trichostrongyle eggs; they are much larger, brownish in colour and have parallel sides (Figure 13.3).
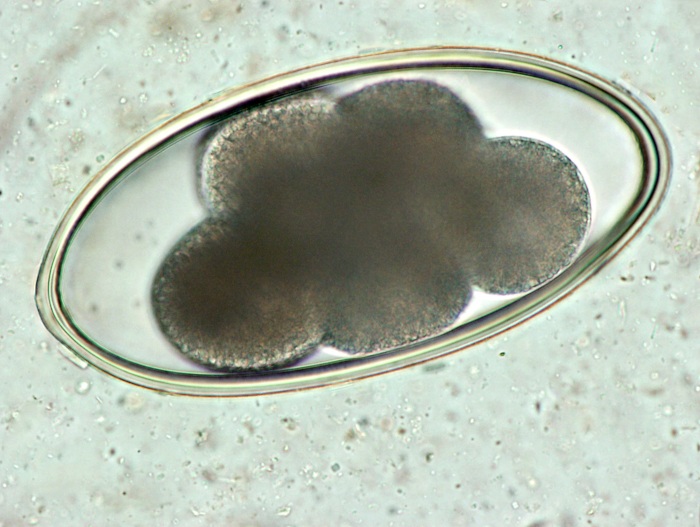
Figure 13.3: Microphotograph of a Nematodirus egg. Photo: Cosme Sánchez-Miguel.
As the majority of damage with acute nematodirosis occurs in the pre-patent period and this parasite is known to be a relatively poor egg producer (DAFM 2018), caution must be exercised when interpreting faecal egg count data. Accordingly, we note that although >85 per cent of faecal samples tested over the course of 2018 yielded negative results, this should not be considered evidence of freedom from infestation for the majority of those tested (Table 13.2.
| Result | No. of samples | Percentage |
|---|---|---|
| Negative | 1431 | 85.5 |
| Low (50-500 epg) | 200 | 12.0 |
| Medium (500-1200 epg) | 29 | 1.7 |
| High (>1200 epg) | 13 | 0.8 |
Nonetheless, it is worth noting that thresholds dictating medium and high parasitic burdens are much lower for Nematodirus than for other gastrointestinal nematodes, >150 epg and >300 epg respectively. Detection of such burdens in faeces of lambs of a certain age are likely to be significant. Furthermore, presence of such burdens in animals which have recently received anthelmintic treatment should also raise concern as Nematodirus battus has traditionally shown very little evidence of resistance to Group 1 (Benzimidazole) anthelmintics, prior to the first case being reported in 2011 (Abbott K.A. 2012).
The lowest proportions of samples testing positive were observed from December to March; tying with expected seasonality and life cycle. The synchronised mass hatch at the end of April in 2018 (approximately two weeks later than usual due to a particularly cold spring) resulted in a small increase in April (Figures 13.4 and 13.5). Substantial increases were then seen in May and June, as higher numbers of parasites matured and commenced laying eggs. Finally, smaller increases observed in September and October may link in with a second, smaller, seasonal peak emerging in the annual disease pattern.
The largest numbers of samples were analysed in early summer and mid autumn, suggesting good awareness of when nematodirosis may be causing an issue.
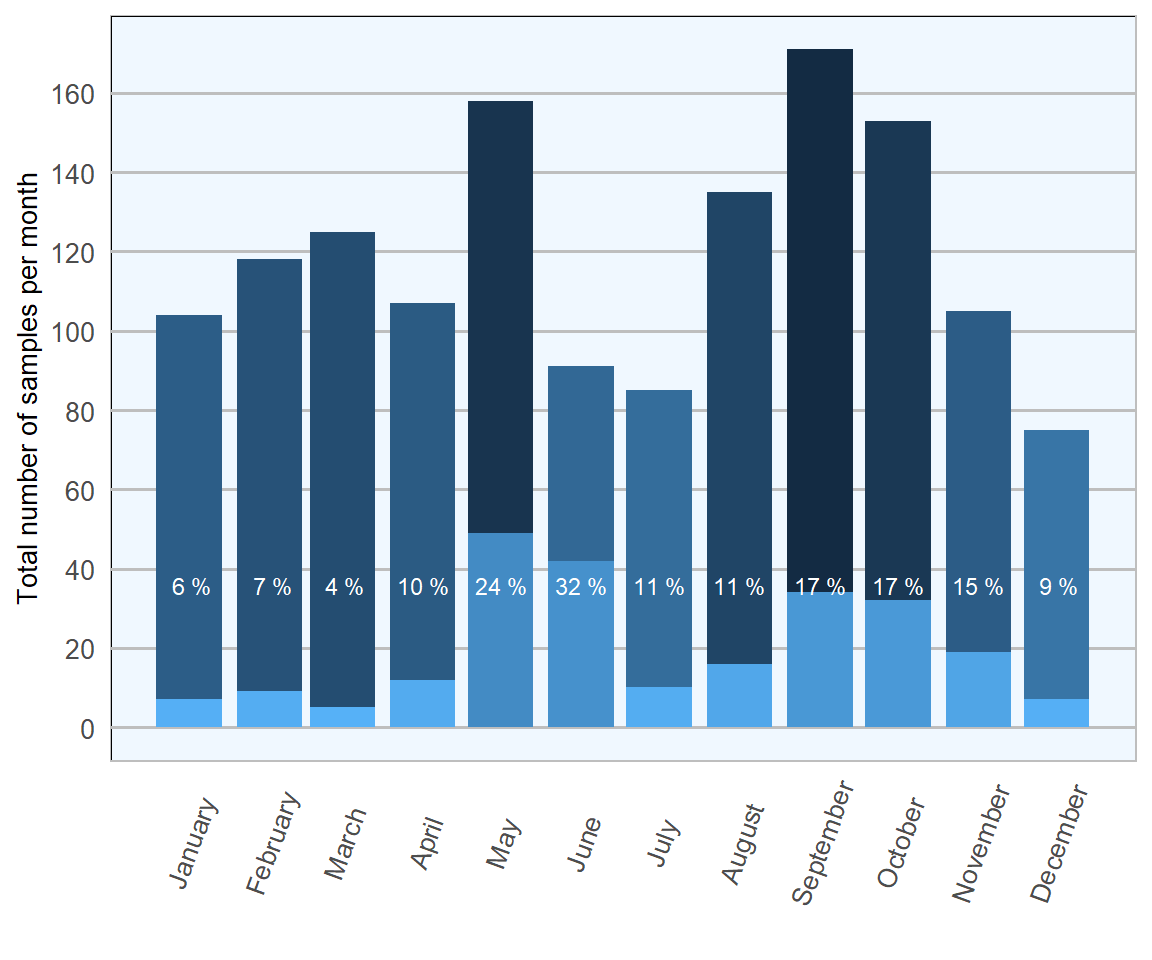
Figure 13.4: Count of ovine faecal samples exmined for Nematodirus eggs in 2018. The percentage in each bar represents the number of positive samples per month (n= 1673 ).
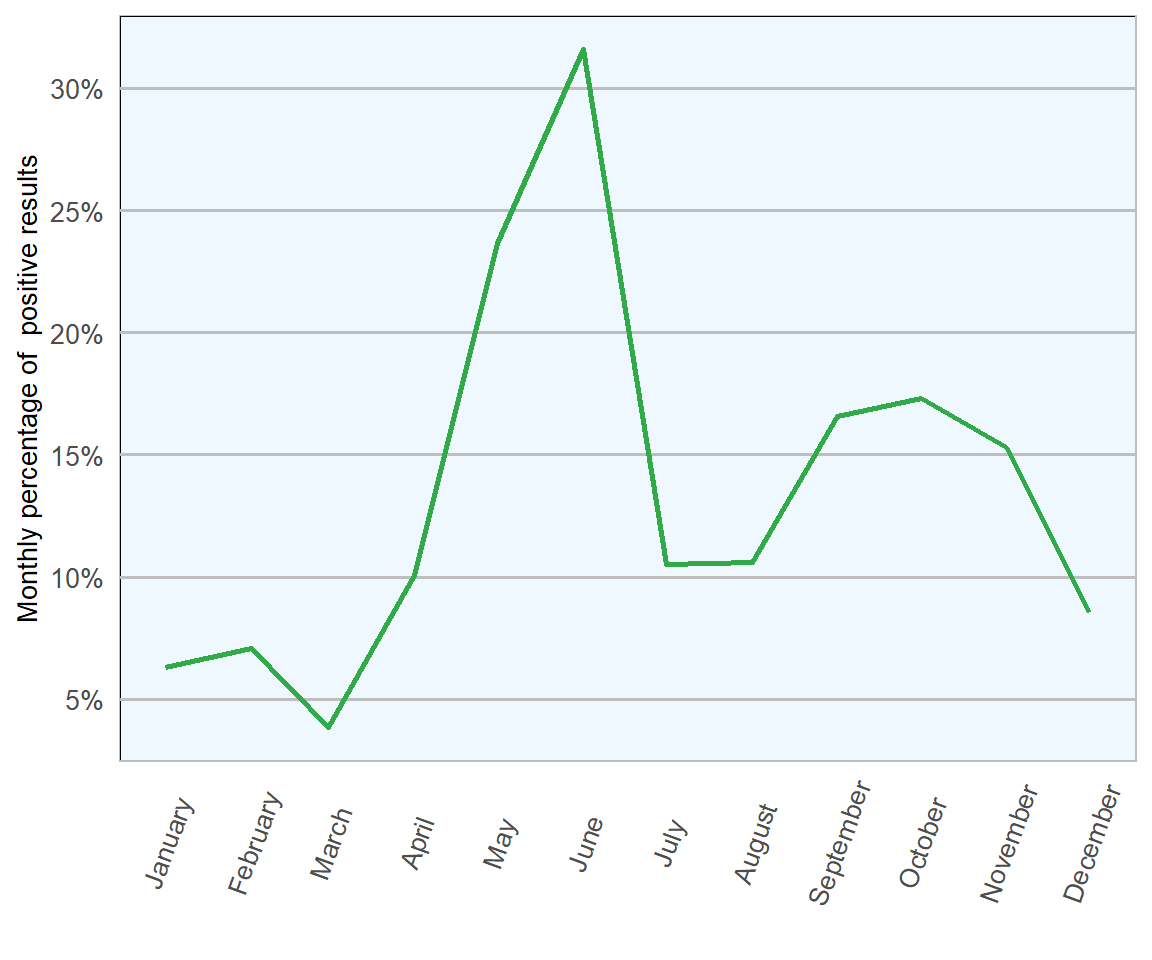
Figure 13.5: Percentage of ovine faecal samples which tested positive for Nematodirus eggs in 2018 (n= 1673 ).
13.4 Coccidia
Coccidia are single celled (protozoan) parasites of the Eimeria spp. Species are host specific and, although there are some 15 different types that infect sheep, only two are believed to cause clinical disease; Eimeria crandallis and Eimeria ovinoidalis. Of these two, the latter is more pathogenic, causing extensive permanent damage to the lining of ileum, caecum and colon.
Disease tends to emerge from April/May onwards, primarily affecting 4–12 week old lambs with peaks at 5–6 weeks when lambs first start to graze. Acute clinical disease is not commonly seen in individuals older than 3-4 months of age (Scott 2012).
Ovine coccidian life cycle takes 12–20 days and differs to nematode life cycle in several ways; the most significant of these relates to the ability of coccidia to multiply internally by asexual and sexual reproduction (Figures 13.6 and 13.7), resulting in the number of oocysts shed being many millions higher than the number ingested, leading to very rapid levels of environmental contamination within very short periods.
| Result | No. of samples | Percentage |
|---|---|---|
| Not Detected | 929 | 55 |
| Light Infection | 478 | 28 |
| Moderate Infection | 176 | 10 |
| Heavy Infection | 73 | 4 |
| Severe Infection | 37 | 2 |
Early in the season, the initial source of infection for lambs comes from the relatively small numbers of parasites shed by nursing ewes and oocysts which have survived over winter in the environment. The multiplier effect means that first infected lambs produce much higher levels of environmental contamination for lambs born later in the season.
Therefore, it is not unusual to see coccidiosis issues worsen over the course of the season, with lambs affected more severely and at a younger age, as the year progresses.
Disease tends to be self-limiting and immunity develops quickly, although there is no cross-protection between different species of coccidia (Scott 2012). Often, survivors of clinical disease suffer permanent damage and fail to return to full health and acceptable growth rates despite successful treatment and management.
For this reason, preventative management is key. As coccidia are ubiquitous in the environment, complete avoidance of disease is considered impractical. Indeed, this may not even be advisable as some studies have shown that early exposure of young lambs to small numbers of parasites may be protective and, therefore, beneficial (Gregory 1995).
As this tends to be an annual and recurrent issue, maintenance of good flock records can help to pinpoint particular risk periods allowing management and/or treatment protocols to be implemented at the most effective times in subsequent years (Scott 2012).
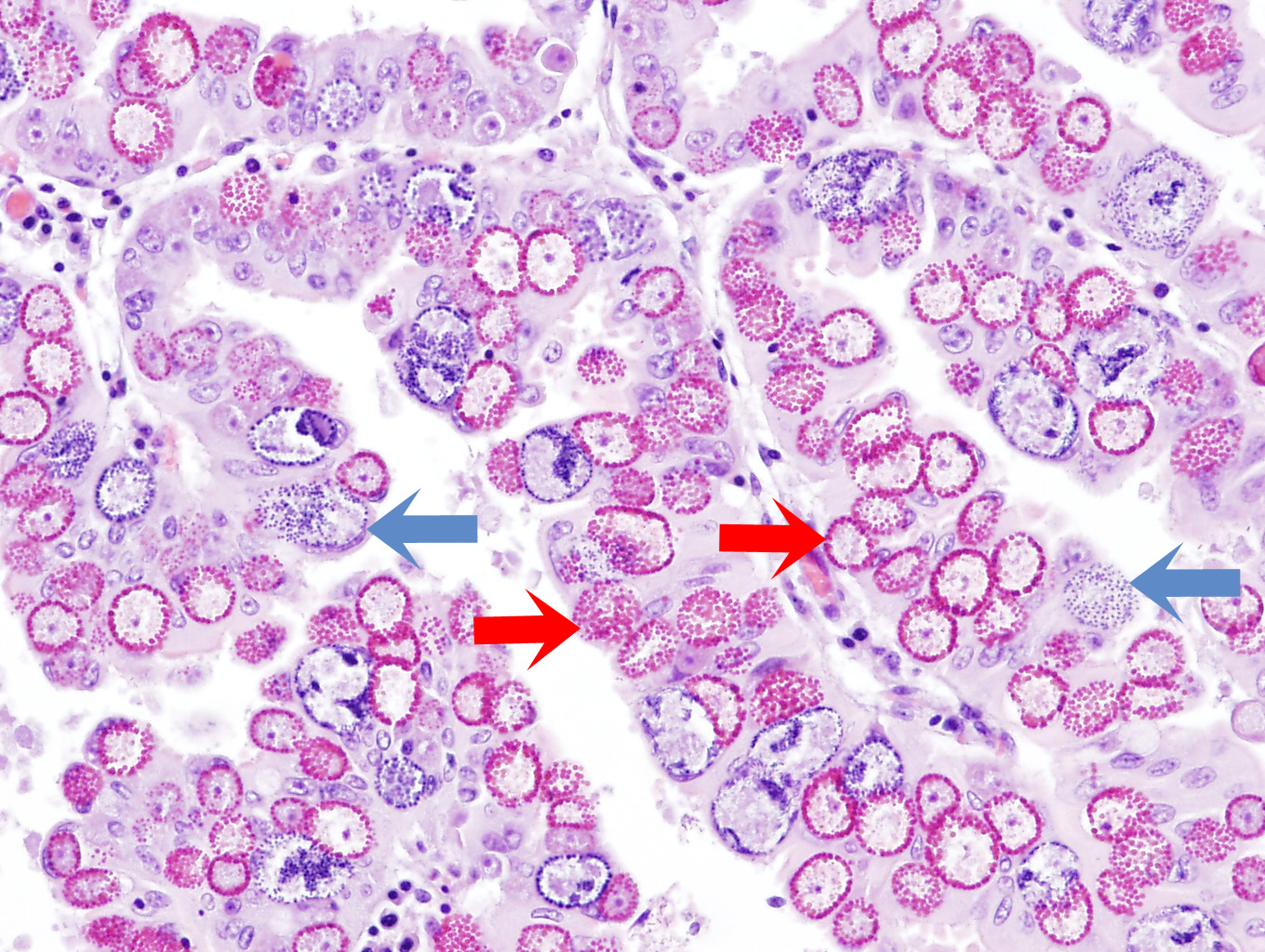
Figure 13.6: Coccidial microgamonts (blue arrow) and macrogamonts (red arrow) in the intestinal villi of an sheep with severe coccidiosis. Photo: Cosme Sánchez-Miguel.
Coccidial oocysts can be identified as pale, transparent, egg-shaped structures, with thin walls and many times smaller than typical trichostrongyle eggs. Large counts can be in excess of 100,000 oocysts per gram.
Unfortunately, as in the case of gastrointestinal nematode infestation, it must be remembered that faecal counts can be limited in several ways. Firstly, as with nematode diseases, acute disease can occur before eggs or oocysts are observed in faeces. Secondly, as coccidial intestinal damage is cumulative, individual faecal oocyst counts may not be at their highest when clinical signs of disease are most pronounced. Furthermore, as the majority of species affecting sheep are not responsible for clinical disease, it is impossible to tell from oocyst counts whether pathogenic species are present and, if so, to what proportion of the count. This renders accurate interpretation of high counts impossible without requesting further testing to speciate the oocysts, which is not routinely conducted.
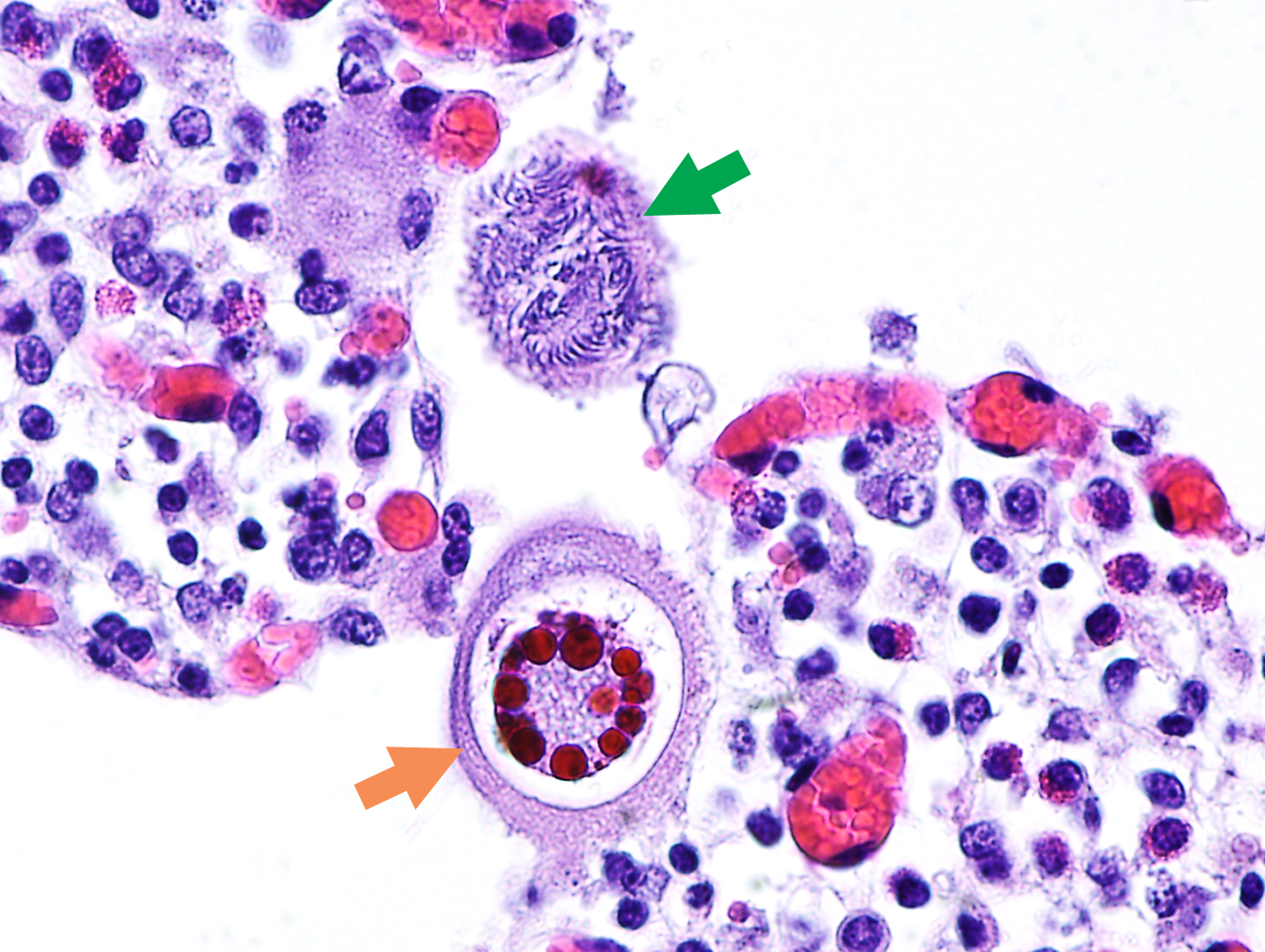
Figure 13.7: Coccidial microgamonts (green arrow) and macrogamonts (orange arrow) in the intestinal villi of an animal with severe coccidiosis. Photo: Cosme Sánchez-Miguel.
For the above reasons high faecal oocyst counts should always be interpreted with caution and in conjunction with clinical history and signs. It may be more useful to assess oocyst counts as indicators of general flock health rather than as a diagnostic tool for individual animals.
In 2018 (Table 13.3), 1693 ovine faecal samples were examined, 45 per cent were positive for coccidial oocysts and 16 per cent had counts suggestive of moderate to severe infestations. This is just over double the proportion of positive samples and almost treble the number of substantial counts than were seen with bovine samples examined during the same year. However, when comparing 2018 RVL figures with 2016 All-Ireland ADSR, there is a suggestion that proportion of positive samples examined has dropped by about 10 per cent.
In 2018 (Figures 13.8 and 13.9), the highest number of samples tested was in May (205) and the highest proportion of samples testing positive was noted in June (65 per cent), both correlate well with the expected period of highest risk of clinical disease, when the infectious load has had a chance to build up and lambs are still vulnerable to disease, not yet having developed protective immunity. From February to May there is a progressively rapid increase in the proportion of positive samples examined, which may correlate with higher number of coccidia in environment due to repeated cycles of infection. After June, there is a general trend towards a lower proportion of positive results, presumably because immunity levels begin to rise from this point onwards. The lowest number of samples examined and the lowest proportion of samples yielding positive results occurred simultaneously in December, which again is logical, as this is the time of year where significant issues with coccidiosis would not be expected as there are few, if any, individuals of vulnerable age.
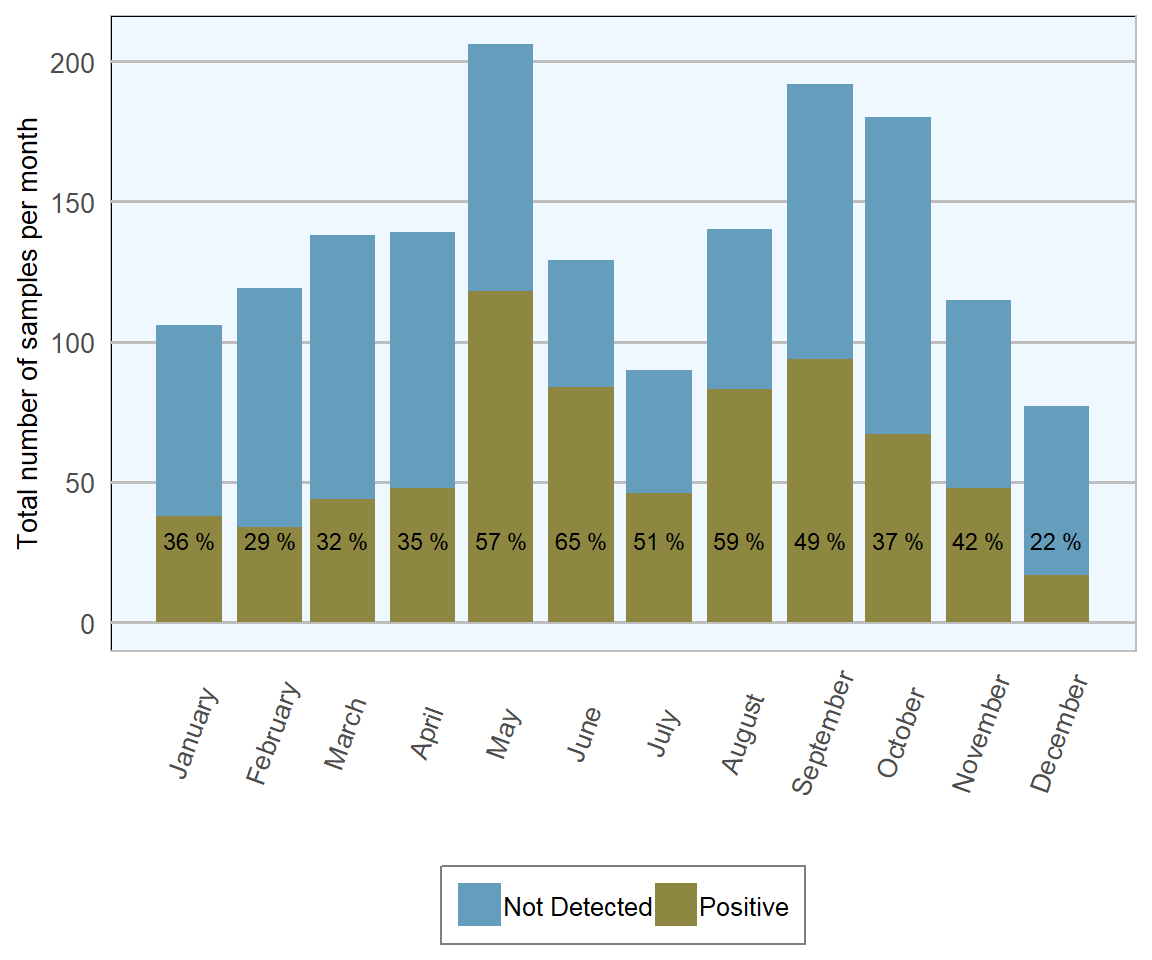
Figure 13.8: Stacked number of ovine faecal samples (all ages) tested for coccidial oocysts in 2018. The percentage in each bar represents the number of positives (n= 1693 ).
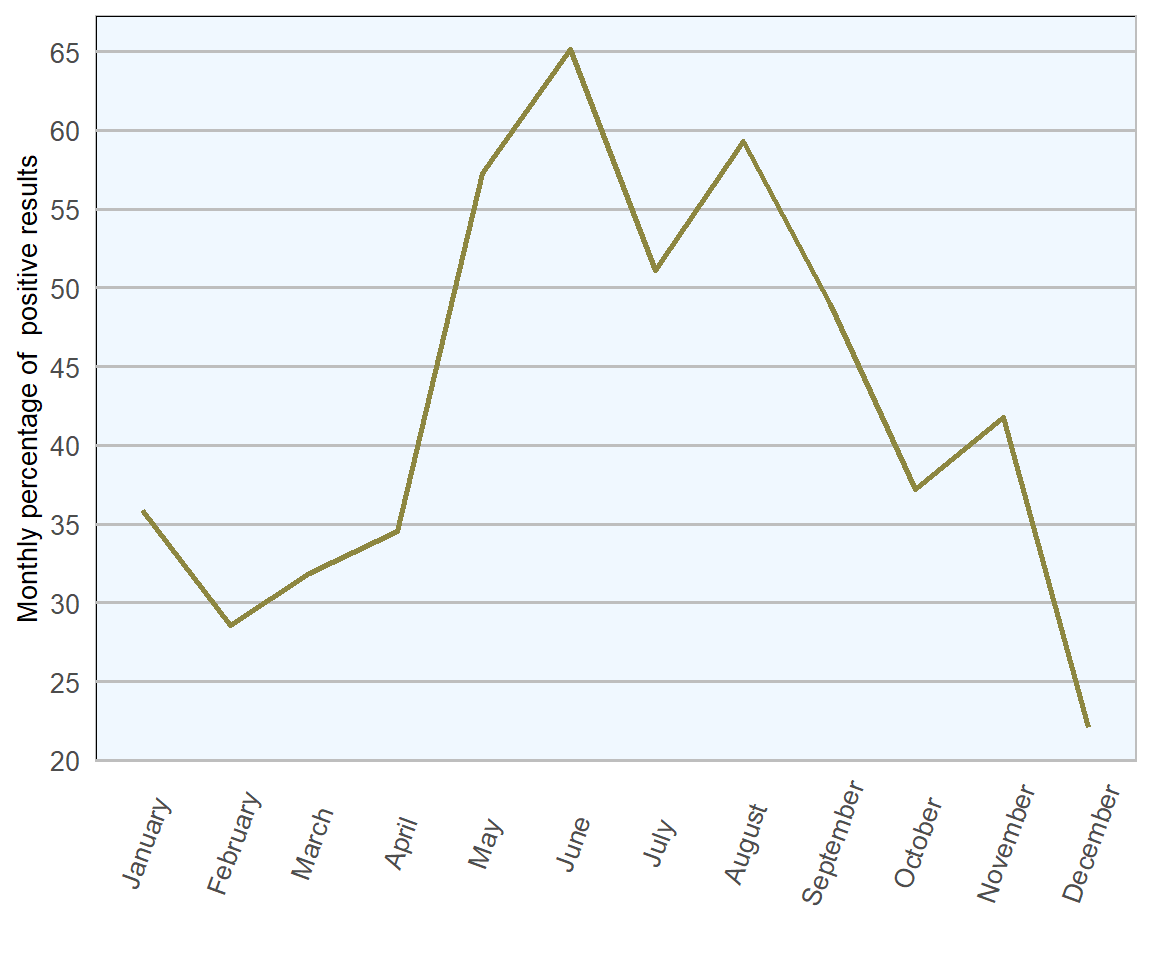
Figure 13.9: Count of ovine faecal samples examined for coccidial oocysts in 2018 (n= 1693 ).
13.5 Liver and rumen fluke
Liver fluke disease in Ireland is associated with Fasciola hepatica, from the family Fasciolidae, that can infest livers of many mammals, including man. This flat leaf-shaped worm reaches an adult length of 25–30 mm and a width of about 13 mm, making it easily recognised during gross inspection of livers. There are two distinct forms of disease recognised; acute (Figure 13.10) and chronic. Both forms are not mutually exclusive and can be present in tandem within the same individual. In addition to the classic signs of disease outlined below, liver fluke has been shown to affect ewe fertility and milk yield, with consequential effects on flock economics (Hynes 2010).
The most commonly identified rumen fluke of cattle and sheep in Ireland is Calicophoron daubneyi, from the family Paramphistomidae. Adults are usually 10 mm in length, pink, fleshy, tear-drop shaped and occupy ruminal and reticular surfaces. Rumen fluke infestations have been an increasingly common finding in Irish ruminants over the past decade or so (Manual 2013).
For many years rumen fluke was believed to be of negative or very limited pathogenicity but, over recent years, studies have associated high burdens of rumen fluke with outbreaks of profuse watery foetid diarrhoea, dullness, inappetence and even sudden death in both cattle and sheep, typically in immature stock (Kajugu et al. 2015). This particularly seems to be the case when very high numbers of immature stages are present in the small intestine (principally the duodeunum) without necessarily featuring presence of adults in rumen, nor eggs in faeces. Knowledge of the entire life cycle and epidemiology of rumen fluke is still limited and presence of adult parasites is not believed to be associated with clinical disease.
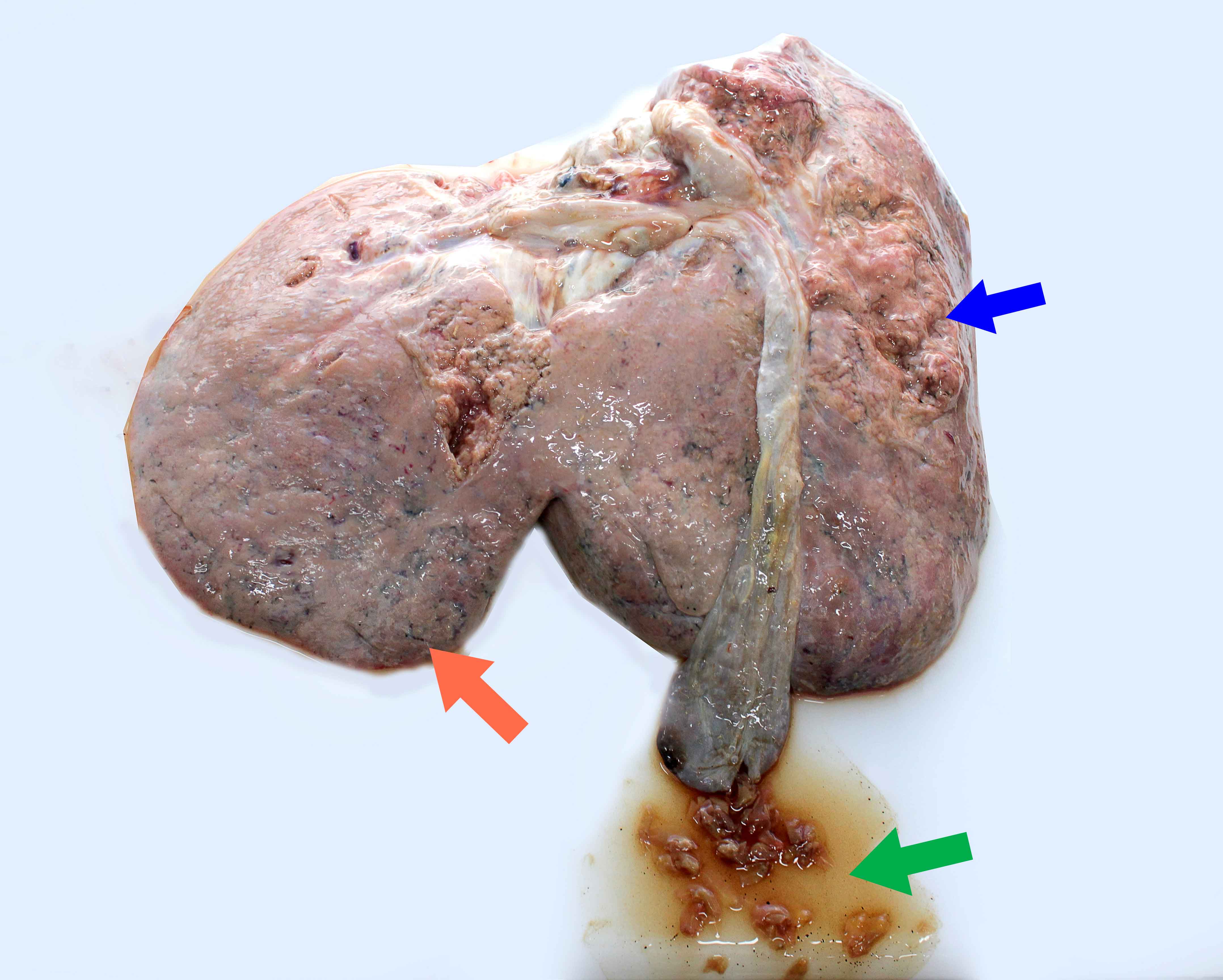
Figure 13.10: Photograph demonstrating characteristic lesions associated with acute faciolosis in the liver of a lamb. Note friable liver (blue arrow), subcapsular haemorrhagic tracts (orange arrow) and trematodes of Fasciola hepatica inside the gallbladder (green arrow). Photo: Cosme Sánchez-Miguel.
The fluke life cycle is protracted with environmental development taking anything from five weeks to three months, depending on underlying weather and temperature conditions. Temperatures greater than are required for fluke eggs to hatch and for the mud snail intermediate host (Galba truncatula) to become active. Pre-patent period, from infection to commencement of egg laying, usually takes 9–12 weeks.
| Result | No. of samples | Percentage |
|---|---|---|
| Liver fluke eggs not detected | 1301 | 90 |
| Positive liver fluke eggs | 140 | 10 |
Classically, acute liver fluke cases would be first seen in mid-late autumn, with chronic cases emerging in winter over the housing period. However, as with many parasitic infestations, traditional epidemiology of liver fluke has changed over recent years. Perhaps climatic pattern fluctuations over the past decade, with milder winters and warmer wetter summers, have encouraged the emergence of new epidemiological variations.
Sheep tend to be more severely affected by liver fluke than cattle, particularly by acute disease. There is little evidence of age related or developmental immunity to liver fluke in either species, meaning adult livestock should be considered equally at risk as youngstock. Presence of liver and rumen fluke can be confirmed by identification of immature or adult stages at post-mortem or by faecal examination to identify presence of eggs. Eggs are of similar appearance but, whilst liver fluke eggs are of a consistent shape with a yellow or gold hue, rumen fluke eggs tend to be more variable in shape and clear in colour.
Presence of fluke eggs in faeces is indicative of presence of adult fluke within bile ducts, and a reasonable indicator for chronic liver fluke disease. However, it is not reliable for identifying cases of disease associated with rumen fluke, nor acute liver fluke disease; for both conditions damage takes place exclusively within the pre-patent period. Furthermore, caution should be taken when interpreting faecal egg counts, as fluke egg counts tend to be low and generally provide a less reliable indicator of overall parasitic burden than most nematode species. In addition, presence of rumen fluke eggs in faeces may not be indicative of disease at all. Therefore, it is again worth emphasising the value of post-mortem examination and abattoir inspections in conjunction with faecal egg count results when investigating suspected fluke outbreaks.
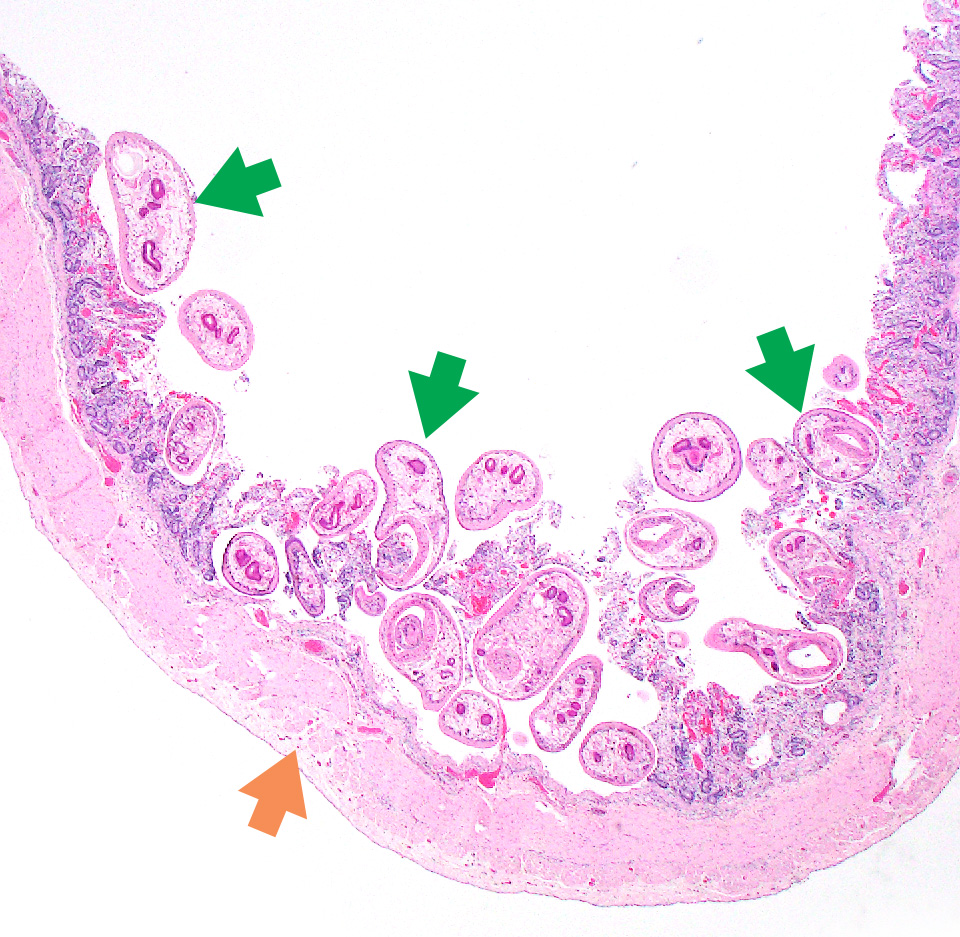
Figure 13.11: Microphotograph of rumen fluke (Calcicophoron daubneyi) in the small intestine. Photo: Seamus Fagan.
In 2018, 10 per cent of 1441 ovine faecal samples examined were positive for liver fluke eggs and 20 per cent were positive for rumen fluke eggs. Once again, comparing 2018 RVL figures with 2016 All-Ireland ADSR (DAFM 2016), this is an improvement from the 2016 figures (14 per cent and 23 per cent respectively). It should be noted that, in 2018, a colder than usual winter/spring followed by an unusually dry summer is perhaps likely to have exerted more of an influence than changes in disease management and control.
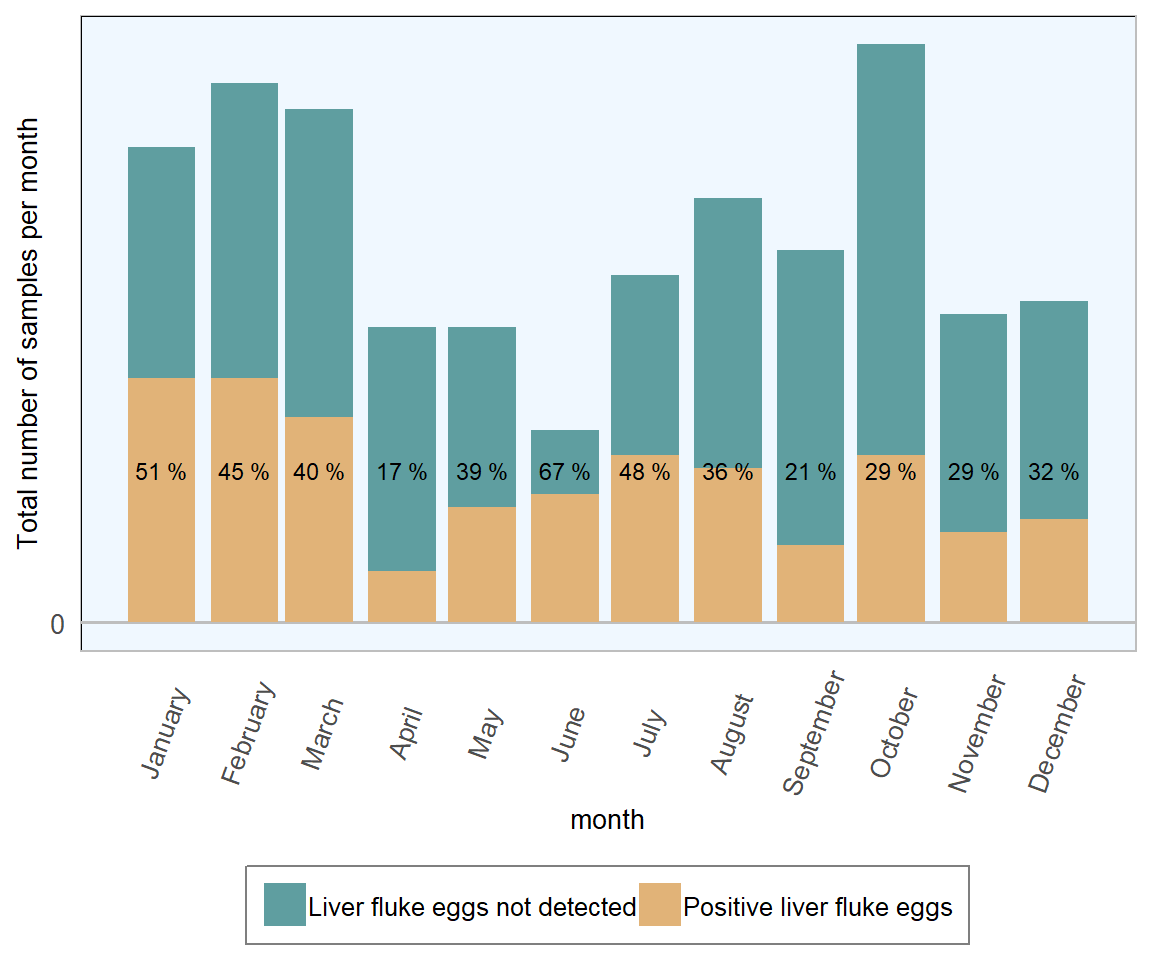
Figure 13.12: Stacked number of ovine faecal samples (all ages) tested for liver fluke in 2018. The percentage in each bar represents the number of positive samples per month (n= 1441 ).
In 2018, mild to moderate monthly fluctuations were see in the proportion of ovine samples testing positive for liver fluke eggs, with the highest proportions seen in the first quarter. This ties in with expectations of the highest risk of chronic disease developing in late winter/early spring, during the housing period.
| Result | No. of samples | Percentage |
|---|---|---|
| Rumen fluke eggs not detected | 1155 | 80 |
| Positive rumen fluke eggs | 286 | 20 |
The monthly fluctuations in rumen fluke positive samples are less easily explained and may refer to as yet unestablished elements of the rumen fluke life cycle and seasonality. As little is currently known about the significance (if any) of chronic rumen fluke infestation in sheep or cattle in Ireland, it is probably not worth commenting further here. However it could be noted that these results suggest that rumen fluke eggs were more commonly found in ovine faecal samples than liver fluke eggs across all months, bar June, in 2018.
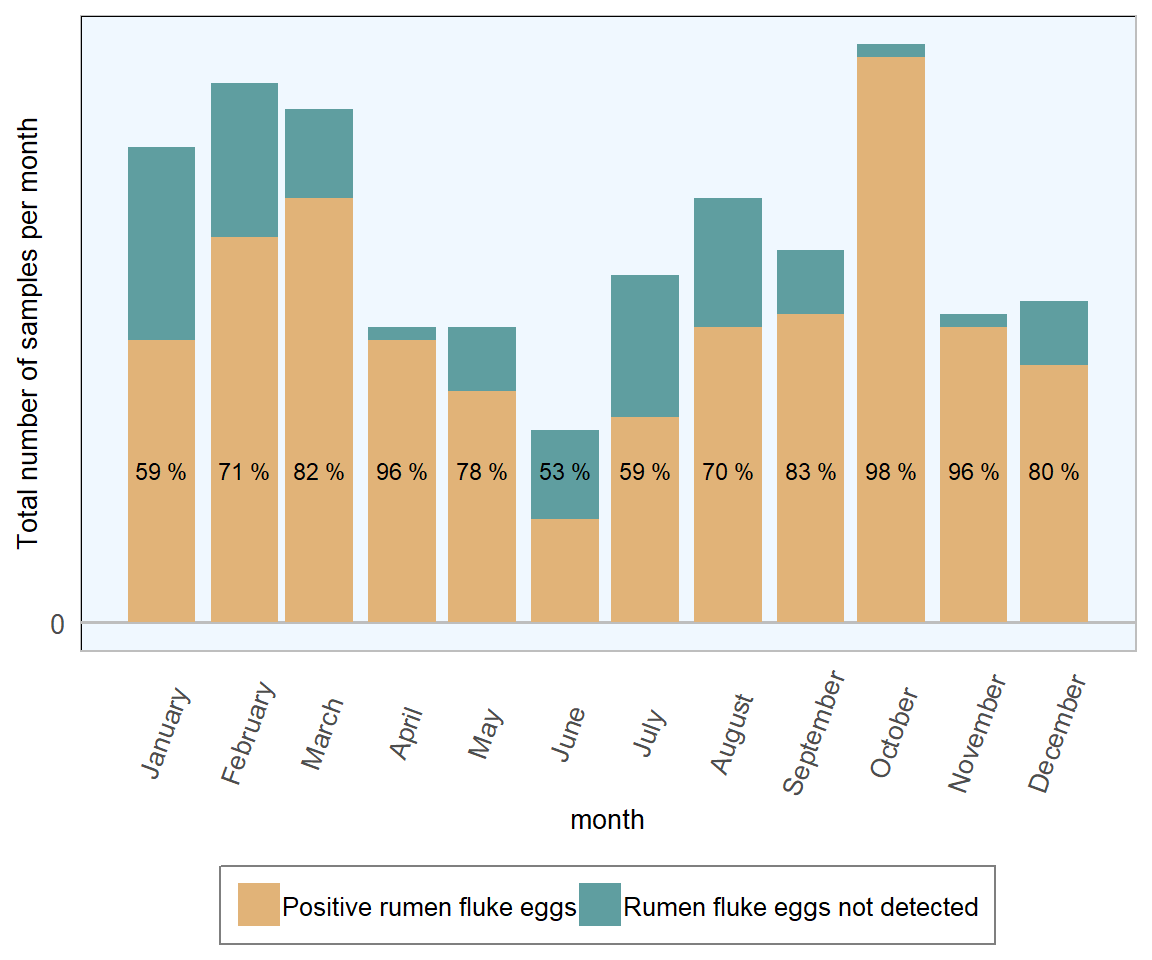
Figure 13.13: Stacked count of ovine faecal samples (all ages) tested for rumen fluke. The percentage in each bar represents positive samples (n= 1441 ).
13.6 Sarcocystosis & Cysticercosis
In an addition to typical ovine parasitic diseases regularly discussed in this report, a combined outbreak of ovine sarcocystosis and cysticercosis came to light in March 2018 as a result of lesions observed at abattoir slaughter.
The increased presence of cyst or pimple-like lesions in lamb carcases from an intensive indoor lamb finishing unit, leading to excessive muscle trimming and carcase condemnations, raised initial concerns. Further laboratory testing involving histopathological and multiplex PCR technology confirmed the involvement of both Taenia ovis (tapeworm) and Sarcocystis tenella (protozoan) (O’Shaughnessy, unpublished data, 2019).
Both parasites utilise dogs as definitive hosts and small ruminants as intermediate hosts. Hosts are seldom significantly impacted clinically by the presence of either parasite. However, sarcocystosis has occasionally been shown to result in reduced flock performance and sporadic ovine abortions (Dubey et al. 2015).
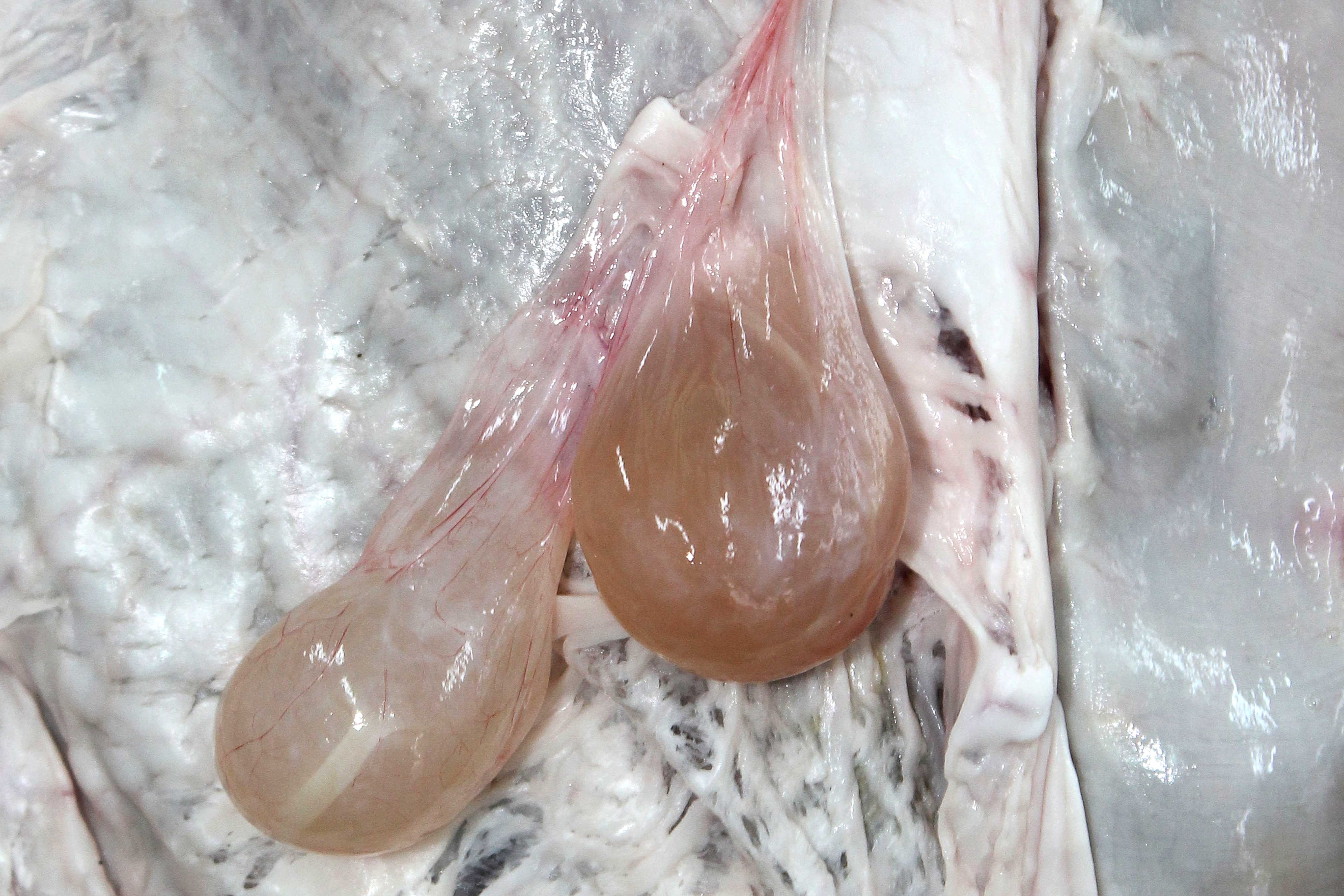
Figure 13.14: Cysticercus tenuicollis in the peritoneal cavity of a sheep. Photo: Cosme Sánchez-Miguel.
In the case of the 2018 outbreak, the scale of carcase condemnation resulted in economic and food safety implications that had to be addressed. On farm investigations revealed risk factors such as suboptimal anthelmintic dosing of farm dogs, inappropriate storage of ovine carcases and carnivore access to feed stores. In addition, excessive pruritus, due to Psoroptes ovis (sheep scab), and lameness were also identified; these had not previously come to light and were identified during farm visits.
This case clearly highlights that indoor lamb finishing units, which are a novel adaptation of sheep farming in Ireland, may be subject to variations in disease prevalence and presentation and should invest in robust flock-health planning with ongoing veterinary oversight to ensure maintenance of acceptable flock health and welfare levels.
References
Abbott K.A., Stubbings L.A., Taylor M. 2012. “SCOPS Technical Manual.” https://www.scops.org.uk/workspace/pdfs/scops-technical-manual-4th-edition-updated-september-2013.pdf.
DAFM. 2016. “All-Island Animal Disease Surveillance Report.” Dept. of Agriculture, Food; the Marine. https://www.agriculture.gov.ie/media/migration/animalhealthwelfare/labservice/rvlreports/AIDSRReport016230118.pdf.
McMahon, Connor, Hillary W. J. Edgar, Jason P. Barley, Robert E. B. Hanna, Gerard P. Brennan, and Ian Fairweather. 2017. “Control of Nematodirus Spp. Infection by Sheep Flock Owners in Northern Ireland.” Irish Veterinary Journal 70 (1): 31. doi:10.1186/s13620-017-0109-6.
Dijk, J. van, and E. R. Morgan. 2008. “The Influence of Temperature on the Development, Hatching and Survival of Nematodirus Battus Larvae.” Parasitology 135 (2). Cambridge University Press: 269–83. doi:10.1017/S0031182007003812.
DAFM. 2018. “2018 Nematodirus Forecast.” https://www.agriculture.gov.ie/press/pressreleases/2018/april/title,116630,en.html.
Scott, P. 2012. “Coccidiosis in Sheep.” https://www.nationalsheep.org.uk/workspace/news-pdfs/12-03-Coccidiosis-in-Sheep(E)29032012122058.pdf.
Gregory, M. W. 1995. “Prevention of Coccidiosis in Lambs.” Edited by. In Practice 17 (8). British Medical Journal Publishing Group: 382–82. doi:10.1136/inpract.17.8.382-a.
Hynes, F. 2010. “Internal Parasites: An Ongoing Problem on Sheep Farms.” https://www.teagasc.ie/media/website/publications/2010/InternalParasites.pdf.
Manual, COWS Technical. 2013. “Control of Liver and Rumen Fluke in Cattle.” https://www.cattleparasites.org.uk/app/uploads/2018/04/Control-liver-and-rumen-fluke-in-cattle.pdf.
Kajugu, P.E., R.E.B. Hanna, H.W. Edgar, C. McMahon, M. Cooper, A. Gordon, J.P. Barley, F.E. Malone, and I. Brennan G.P.and Fairweather. 2015. “Fasciola Hepatica: Specificity of a Coproantigen Elisa Test for Diagnosis of Fasciolosis in Faecal Samples from Cattle and Sheep Concurrently Infected with Gastrointestinal Nematodes, Coccidians and/or Rumen Flukes (Paramphistomes), Under Field Conditions.” Veterinary Parasitology 212 (3): 181–87. doi:https://doi.org/10.1016/j.vetpar.2015.07.018.
Dubey, J.P., R. Calero-Bernal, B.M. Rosenthal, C.A. Speer, and R. Fayer. 2015. Sarcocystosis of Animals and Humans. Second Edition. CRC Press.
A cooperative effort between the VLS and the SAT Section of the DAFM