
Section 5 Zinc Sulphate Turbidity (ZST) Test
- Iam Hogan
- Research Officer, Limerick Regional Veterinary Laboratory, Knockalisheen, Limerick, Ireland
The Zinc Sulphate Turbidity (ZST) test is an indirect measurement of passive transfer of immunoglobulins via colostrum from the dam to the neonate. The adequate delivery of good quality colostrum is an important part of calf management, as transfer of immunity provides protection to neonates from common infectious diseases that contribute to illness and death.
5.1 ZST test and the importance of colostrum
Failure of passive transfer (FPT) is best assessed on a herd basis. It is recommended to sample several healthy calves or lambs, up to twelve animals less than a week old. Blood sampling should not be done on the first day of life as peak circulating immunoglobulin is achieved 36 hours after colostrum ingestion.
The ZST test used in the DAFM laboratory service was developed by McEwan et al. (1970), who determined that metal salts such as Zinc Sulphate would be precipitated from solution in proportion to the level of immunoglobulin present in a serum sample, once the two are combined. In recent years, to improve the quality of this test, a higher concentration of Zinc Sulphate solution has been used (Hudgens et al. 1996).
| Submission type | Status | No. of samples | Mean | Percentage |
|---|---|---|---|---|
| Diagnostic | Optimal | 588 | 33.0 | 72 |
| Adequate | 120 | 16.4 | 15 | |
| Inadequate | 106 | 7.4 | 13 | |
| Carcass | Optimal | 132 | 29.7 | 34 |
| Adequate | 89 | 15.5 | 23 | |
| Inadequate | 172 | 6.4 | 44 |
5.2 Outline of 2018 figures
In 2018, 814 blood samples were submitted for Zinc Sulphate Turbidity Test for diagnosic purposes, i.e. from live animals. Table 5.1 and Figure 5.1 show that 72 per cent of samples submitted for diagnostic purposes were in the optimal range, i.e. had a ZST result greater than or equal to 20 units; 15 per cent were within the adequate range, ZST results between 12.5 and 20 units, and the remaining 13 per cent were in the inadequate range, ZST results below 12.5 units. The distribution of ZST values is charted in Figure 5.2.
2018 figures are a marked improvement on just a few years ago, for example in 2014 only 51 per cent of diagnostic samples returned a value greater than or equal to 20 units. There are two likely reasons for this improvement: information campaigns conducted by several bodies to impress upon herd-owners the importance of good colostrum management, which are likely to have led to improved colostrum feeding practices, and better targeted testing of calves to evaluate colostrum management.
Measurement of serum total protein is another way to assess for failure of passive transfer (FPT). This test is useful for monitoring colostrum management in healthy calves, but it is not suitable for sick, dehydrated or dying calves. The analysis can be carried out either in farms with a refractometer (Figure 5.3, or in veterinary clinics using an in-house biochemistry analyser (Bielmann et al. 2010). When used for screening, 80 per cent of samples should show values above 55 g/l.
5.3 Shortcomings in submission practices
The optimum way to investigate FPT is on a herd basis. Single samples are not ideal as individual results can vary and may not be reflective of herd incidence of FPT. Colostrum management should be examined on a herd basis; when assessing a herd, the proportion of calves in the herd which have received inadequate colostral immunity is of more significance than the average serum immunoglobulin concentration.
Currently, DAFM laboratories determine the immune status of calves by ZST tests on an on-demand basis. Submissions overwhelmingly consist of a sample from a single calf; in 2018, 41 per cent of submissions for ZST testing contained one single sample and only 23 per cent of submissions contained 5 or more samples. This proportion has improved over the last few years; in 2014 single samples made up 79 per cent of submissions while only 7 per cent of submissions contained five or more samples. Awareness of the importance of colostrum in herd health, and of the need for planned investigations into the efficacy of colostrum feeding, has increased.
Clinical history provided in the laboratory submission forms is in many cases minimal but one would suspect many single samples come from sick calves. Samples from sick calves are not suitable to evaluate colostrum management as disease processes will affect circulating immunoglobulin. Immunoglobulin will be lost from circulation as it binds with antigen, or through protein-losing conditions such as enteropathy and nephropathy; dehydration, on the other hand, may lead to artificially high ZST results through haemoconcentration.
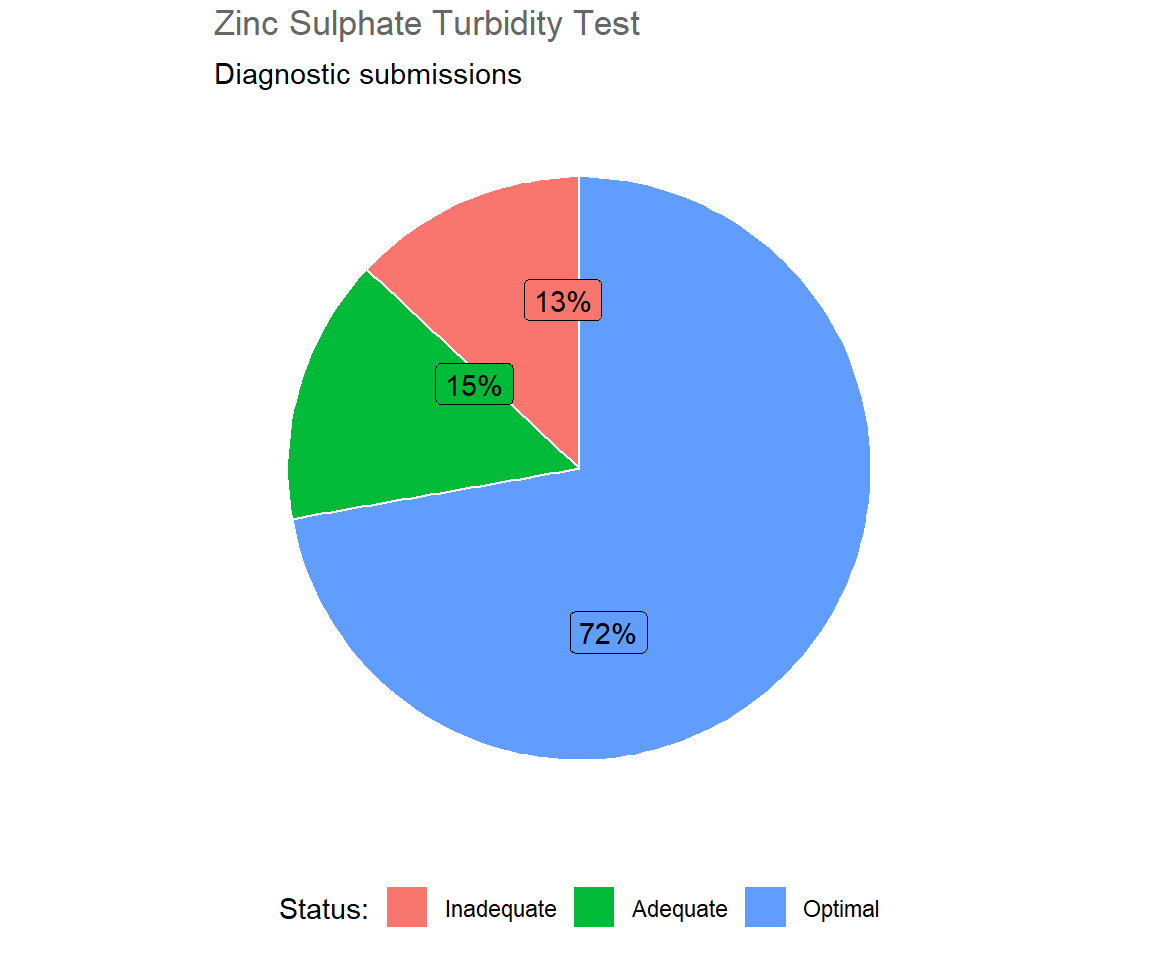
Figure 5.1: Results of ZST from submitted bovine blood samples in 2018 (n= 814 ).
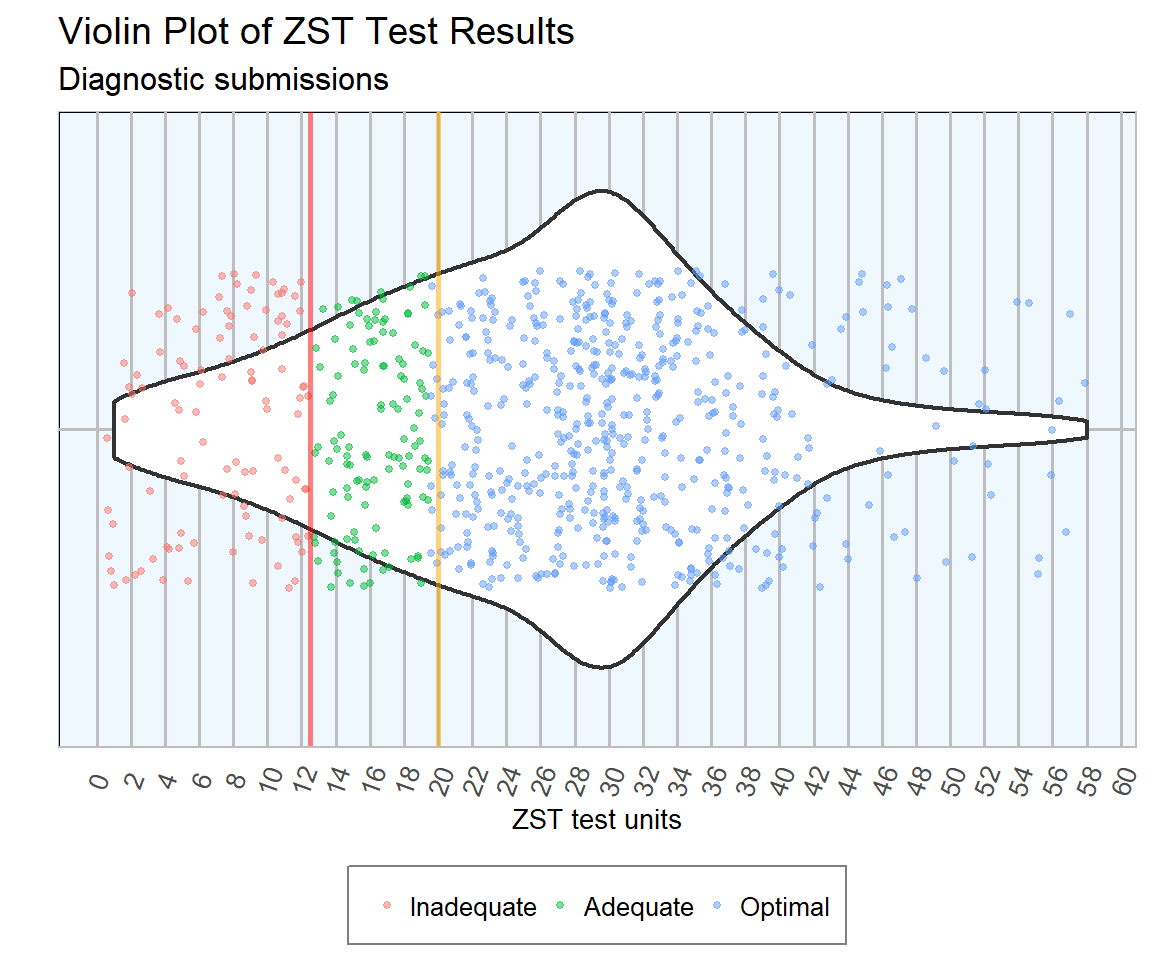
Figure 5.2: Distribrtion of ZST test results during 2018. Optimal colostral immunity is defined as greater than 20 units (orange line), adequated between 12.5 and 20 units and inadequated less than 12.5 units (red line). The width of the white area at each point of the x axis is proportional to the number of samples returning a ZST result of that value. Outliers with values greater than 60 units (24 samples) were removed from the plot (n= 790 ).
5.4 ZST and immunoglobulin classes
ZST tests give results which correlate well with levels of total immunoglobulin and with IgG, which is understandable as IgG comprises the largest proportion of immunoglobulin in both colostrum and the bloodstream of calves drinking colostrum. Results from the ZST test do not give as good a measure of circulating immunoglobulin M (IgM), which composes a smaller proportion of the immunoglobulin in colostrum and is important in the prevention of septicaemia. To complicate matters, IgM molecules are commonly much larger than those of IgG and closure of the intestine to IgM, in other words the point at which the intestinal mucosa ceases absorbing IgM intact into the blood stream, occurs much earlier than it does for IgG.
The upshot of this is that a calf receiving colostrum after a slight delay may have adequate, or even optimal, levels of IgG and total immunoglobulin, yet have absorbed inadequate levels of IgM. This inadequacy will not be reflected in ZST results or in results from any of the alternative indirect tests for FPT, such as total protein or Gamma-Glutamyl Transferase (GGT) levels. Only a direct test for IgM, such as an ELISA or radial immunodiffusion (RID), will pick up this deficiency.

Figure 5.3: Brix refractometer. Colostrum quality can be assessed by placing a drop of colostrum in the stage and looking through the eyepiece. Photo: Cosme Sánchez-Miguel.
5.5 Post mortem samples
Another source of samples for ZST testing in DAFM laboratories is blood harvested from calves at necropsy, which by definition are not from healthy calves. ZST results from calves sampled at necropsy, except in the case of very acute deaths, may give misleading results due to the course of illness that preceded death. However, RVL staff may use ZST results from these calves to flag possible cases of FPT in a herd, if results suggestive of FPT are returned this can prompt a possible need to investigate further the performance of colostrum management in that herd. When ZST testing was carried out on 393 samples taken from calf carcasses during post mortem examinations in the laboratory service, 44 per cent of samples had results indicating a failure of passive transfer, a further 23 per cent of samples were in the suboptimal range.
5.6 Ovine submissions
Submissions from ovine neonates for ZST testing are low, especially diagnostic submissions of which only four were received in 2018. Of these, one was suggestive of FPT, but such a small sample size does not make it possible to draw conclusions. Samples were collected from lamb carcasses in higher numbers, and it was found that 96 out of 148 (or 65 per cent) were below the optimal level. Submission rates of ovine neonates for necropsy have been elevated by a study into sheep mortality which was conducted in a number of RVLs in 2018. We must again bear in mind the possible limitations of this test when performed on samples taken from dead animals. It is likely that failure of passive transfer in lambs is most commonly due to mis-mothering, the risk may be also high for lambs born as triplets.
While lambs and calves from beef breeds will usually receive adequate colostrum by suckling, dairy calves require herdowner intervention in order to consume enough colostrum to give sufficient protection. This is due to a dilution effect on colostrum quality caused by the higher volumes of milk produced by modern dairy cows. Ideally, the first feed of colostrum should be given to the calf within two hours of birth and certainly no later than six hours after birth. The quantity required should be based on weight, with the typical 35–45 kg dairy calf needing 3 l and smaller cross-bred calves needing less. Poor transfer of colostral immunity may be due to poor quality colostrum, low colostral intake, poor colostrum absorption or a combination of these three factors.
Supplementary feeding using a stomach tube or oesophageal feeder may be necessary. Frozen colostrum may be used when necessary. Artificial colostrum is less effective but may be used as a last resort. It should always be remembered that improved colostrum feeding practices will not completely compensate for inadequate hygiene.
References
McEwan, A D, E W Fisher, I E Selman, and W J Penhale. 1970. “A Turbidity Test for the Estimation of Immune Globulin Levels in Neonatal Calf Serum.” Clinica Chimica Acta; International Journal of Clinical Chemistry 27 (1): 155–63.
Hudgens, K A, J W Tyler, T E Besser, and D S Krytenberg. 1996. “Optimizing Performance of a Qualitative Zinc Sulfate Turbidity Test for Passive Transfer of Immunoglobulin G in Calves.” American Journal of Veterinary Research 57 (12): 1711–3.
Bielmann, V., J. Gillan, N.R. Perkins, A.L. Skidmore, S. Godden, and K.E. Leslie. 2010. “An Evaluation of Brix Refractometry Instruments for Measurement of Colostrum Quality in Dairy Cattle.” Journal of Dairy Science 93 (8): 3713–21. doi:https://doi.org/10.3168/jds.2009-2943.
A cooperative effort between the VLS and the SAT Section of the DAFM