Useful functions for biometrical models
Tiago Olivoto
2023-03-06
Source:vignettes/vignettes_biometry.Rmd
vignettes_biometry.RmdSee the section Rendering engine to know how HTML tables were generated.
Find possible outliers in a data frame
The function find_outlier() may be used to identify
possible outliers in a dataframe. It is suggested that before applying
any statistical procedures, outliers be checked out.
library(metan)
data_out <- data_ge2
data_out[12, 4] = 5
find_outliers(data_out, var = PH, plots = TRUE)
# Trait: PH
# Number of possible outliers: 1
# Line(s): 12
# Proportion: 0.6%
# Mean of the outliers: 5
# Maximum of the outliers: 5 | Line 12
# Minimum of the outliers: 5 | Line 12
# With outliers: mean = 2.499 | CV = 15.58%
# Without outliers: mean = 2.483 | CV = 13.46%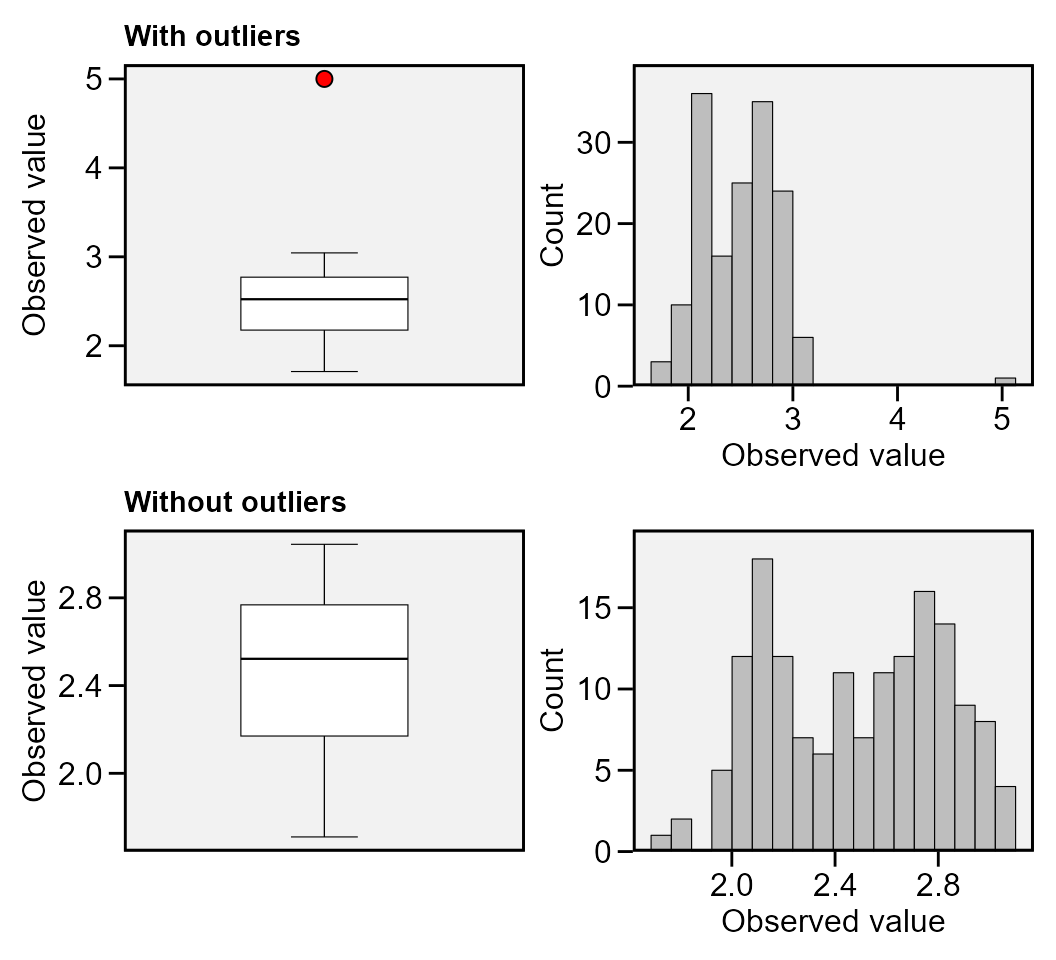
To check the outliers in different levels of a factor, use the
argument by. As an example, we will find possible outliers
for each level of the factor ENV.
find_outliers(data_ge2, PH, by = ENV)
# No possible outlier identified.
#
# No possible outlier identified.
#
# Trait: PH
# Number of possible outliers: 4
# Line(s): 7 11 14 15
# Proportion: 11.4%
# Mean of the outliers: 2.438
# Maximum of the outliers: 2.766 | Line 11
# Minimum of the outliers: 1.71 | Line 7
# With outliers: mean = 2.167 | CV = 10.309%
# Without outliers: mean = 2.136 | CV = 7.377%
#
# No possible outlier identified.
# ENV outliers
# 1 A1 0
# 2 A2 0
# 3 A3 4
# 4 A4 0To group by more than one variable, use the function
group_by() is used.
data_ge2 %>%
group_by(ENV) %>%
find_outliers(PH)
# No possible outlier identified.
#
# No possible outlier identified.
#
# Trait: PH
# Number of possible outliers: 4
# Line(s): 7 11 14 15
# Proportion: 11.4%
# Mean of the outliers: 2.438
# Maximum of the outliers: 2.766 | Line 11
# Minimum of the outliers: 1.71 | Line 7
# With outliers: mean = 2.167 | CV = 10.309%
# Without outliers: mean = 2.136 | CV = 7.377%
#
# No possible outlier identified.
# ENV outliers
# 1 A1 0
# 2 A2 0
# 3 A3 4
# 4 A4 0Correlations
Linear and partial correlation coefficients
Pearson’s linear correlation does not consider the influence a set of traits on the relationship between two traits. For example, the hypothetical correlation of r = 0.9 between x and y may be due to the influence of a third trait or group of traits acting together. To identify this linear effect between x and y controlling statistically the effect of others traits, the partial correlation is used. From Pearson’s simple correlation matrix, the partial correlation is calculated by the following equation:
\[ {r_{xy.m}} = \frac{{ - {a_{xy}}}}{{\sqrt {{a_{xx}}{a_{yy}}} }} \]
Where \({r_{xy.m}}\) is the partial correlation coefficient between the traits * x * and * y , excluding the effects of the m * remaining traits of the set; \(- {a_{ij}}\) is the inverse element of the correlation matrix corresponding to xy, \({a_{ii}}{a_{jj}}\) are the diagonal elements of the inverse matrix of correlation associated with trait x and y , respectively. The significance of this correlation is also tested by the test * t * according to the following expression:
\[ t_{calc} = r_{xy.m} \sqrt \frac{n-v}{1-r_{xy.m}^2} \]
Where \(t_{calc}\) is the calculated
Student * t * statistic; $ r_{xy.m} $ is the partial correlation
coefficient for the traits x and y excluding the effect of the other * m
* traits; * n * is the number of observations; and * v * is the number
of traits. Both the linear and partial correlation coefficients may be
obtained using the function lpcor().
lpc1 <-
data_ge2 %>%
select(starts_with("N")) %>%
lpcor()
print_table(lpc1$results)Using the pairs_mantel() function, it is possible to
compute a Mantel’s test (Mantel 1967) for
all pairwise correlation matrices of the above example.
lpc2 %>% pairs_mantel(names = paste("H", 1:4, sep = ""))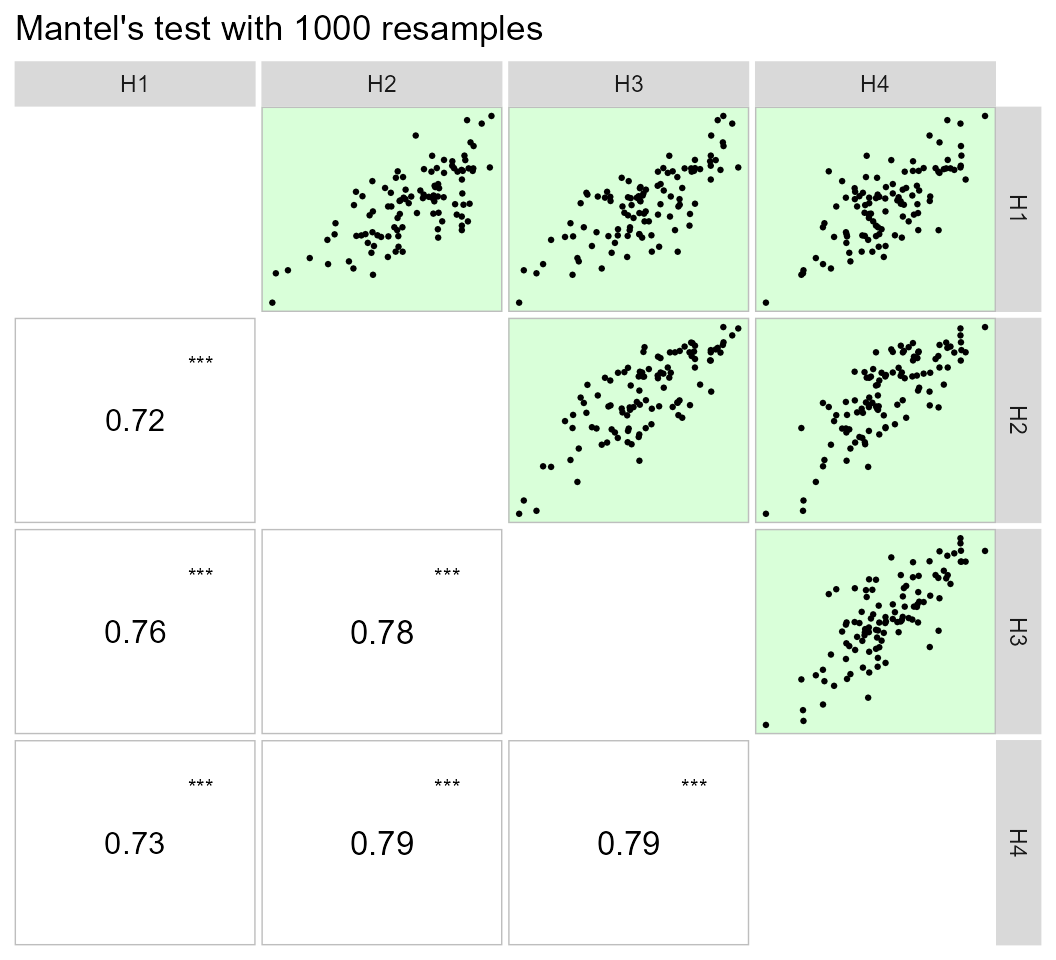
This same plot may be obtained by passing correlation matrices with
the same dimension to an object of class lpcor and then
applying the function pairs_mantel().
as.lpcor(cor(data_ge2[1:30, 5:ncol(data_ge2)]),
cor(data_ge2[31:60, 5:ncol(data_ge2)]),
cor(data_ge2[61:90, 5:ncol(data_ge2)]),
cor(data_ge2[91:120, 5:ncol(data_ge2)]),
cor(data_ge2[121:150, 5:ncol(data_ge2)])) %>%
pairs_mantel(diag = TRUE,
pan.spacing = 0,
shape.point = 21,
col.point = "black",
fill.point = "red",
size.point = 1.5,
alpha.point = 0.6,
main = "My own plot",
alpha = 0.2)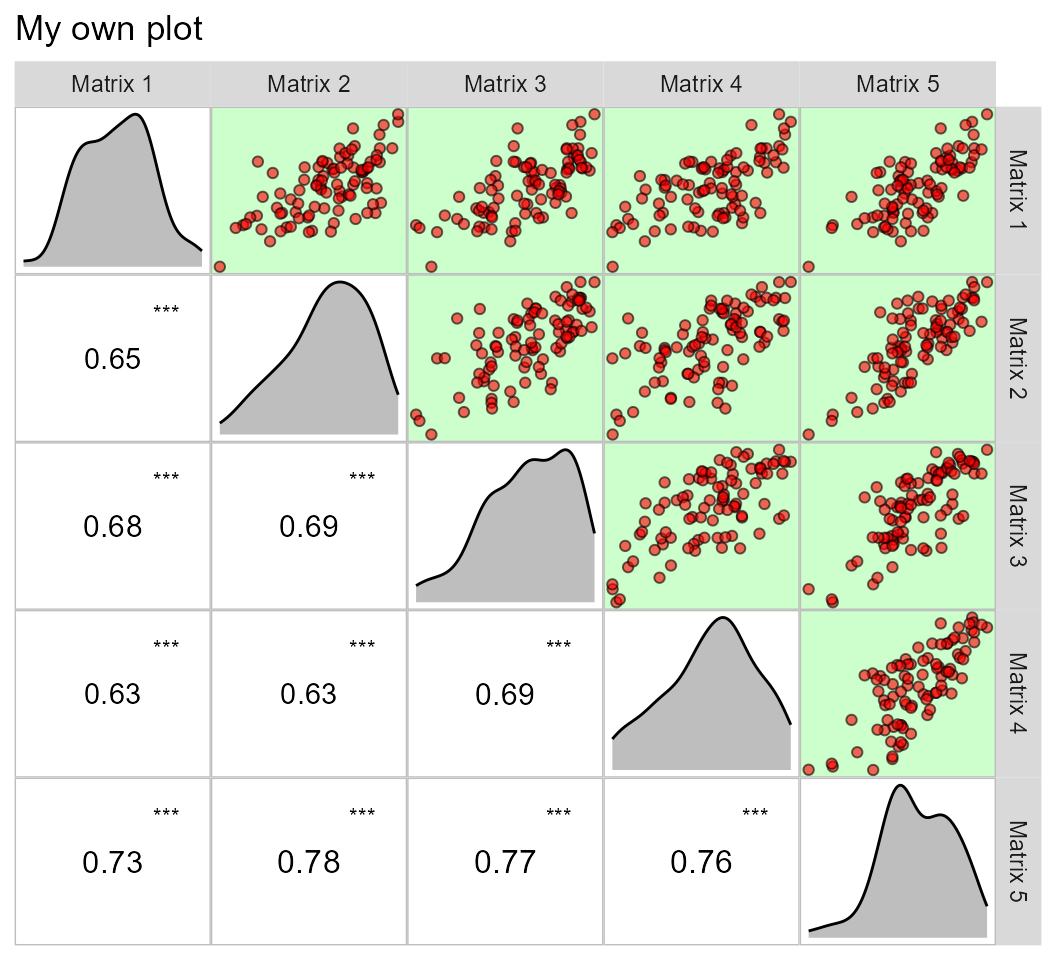
Graphical and numerical visualization of a correlation matrix
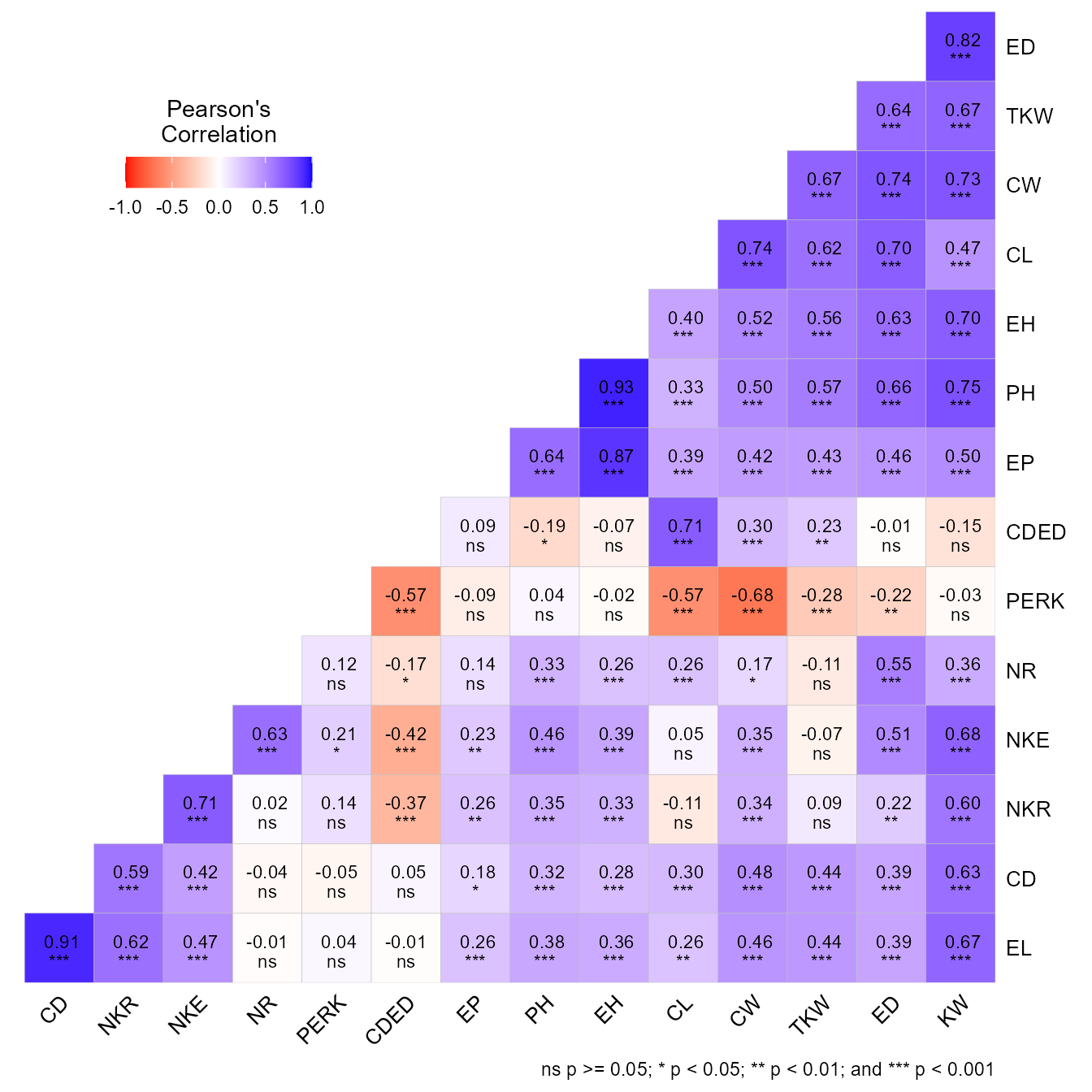
The function corr_coef() can be used to compute Pearson
producto-moment correlation coefficients with p-values. A correlation
heat map can be created with the function plot().
We can use a select helper function to select variables. Here, we
will select variables that starts with “C” OR ends with
“D” using union_var().
ccoef2 <- corr_coef(data_ge2, union_var("C", "D"))
print(ccoef2, digits = 2)
# ---------------------------------------------------------------------------
# Pearson's correlation coefficient
# ---------------------------------------------------------------------------
# CL CD CW CDED ED
# CL 1.00 0.300 0.74 0.708 0.70
# CD 0.30 1.000 0.48 0.045 0.39
# CW 0.74 0.484 1.00 0.300 0.74
# CDED 0.71 0.045 0.30 1.000 -0.01
# ED 0.70 0.390 0.74 -0.010 1.00
# ---------------------------------------------------------------------------
# p-values for the correlation coefficients
# ---------------------------------------------------------------------------
# CL CD CW CDED ED
# CL 0.0e+00 1.4e-04 3.9e-28 4.9e-25 4.7e-24
# CD 1.4e-04 0.0e+00 1.5e-10 5.7e-01 4.9e-07
# CW 3.9e-28 1.5e-10 0.0e+00 1.4e-04 5.3e-28
# CDED 4.9e-25 5.7e-01 1.4e-04 0.0e+00 9.0e-01
# ED 4.7e-24 4.9e-07 5.3e-28 9.0e-01 0.0e+00The function corr_plot() may be used to visualize (both
graphically and numerically) a correlation matrix. Pairwise of
scatterplots are produced and may be shown in the upper or lower
diagonal, which may be seen as a nicer and customizable ggplot2-based
version of the pairs()base R function.
a <- corr_plot(data_ge2, CD, EL, PERK, NKR)
b <-
corr_plot(data_ge2, CD, EL, PERK, NKR,
lower = NULL,
upper = "corr")
c <-
corr_plot(data_ge2, CD, EL, PERK, NKR,
shape.point = 19,
size.point = 2,
alpha.point = 0.5,
alpha.diag = 0,
pan.spacing = 0,
diag.type = "boxplot",
col.sign = "gray",
alpha.sign = 0.3,
axis.labels = TRUE)
d <-
corr_plot(data_ge2, CD, EL, PERK, NKR, CW, NKE,
prob = 0.01,
shape.point = 21,
col.point = "black",
fill.point = "orange",
size.point = 2,
alpha.point = 0.6,
maxsize = 4,
minsize = 2,
smooth = TRUE,
size.smooth = 1,
col.smooth = "black",
col.sign = "cyan",
col.up.panel = "black",
col.lw.panel = "black",
col.dia.panel = "black",
pan.spacing = 0,
lab.position = "tl")
arrange_ggplot(a, b, c, d, tag_levels = "a")It is also possible to use a categorical variable of the data to map the scatterplot by colors.
corr_plot(iris, col.by = Species)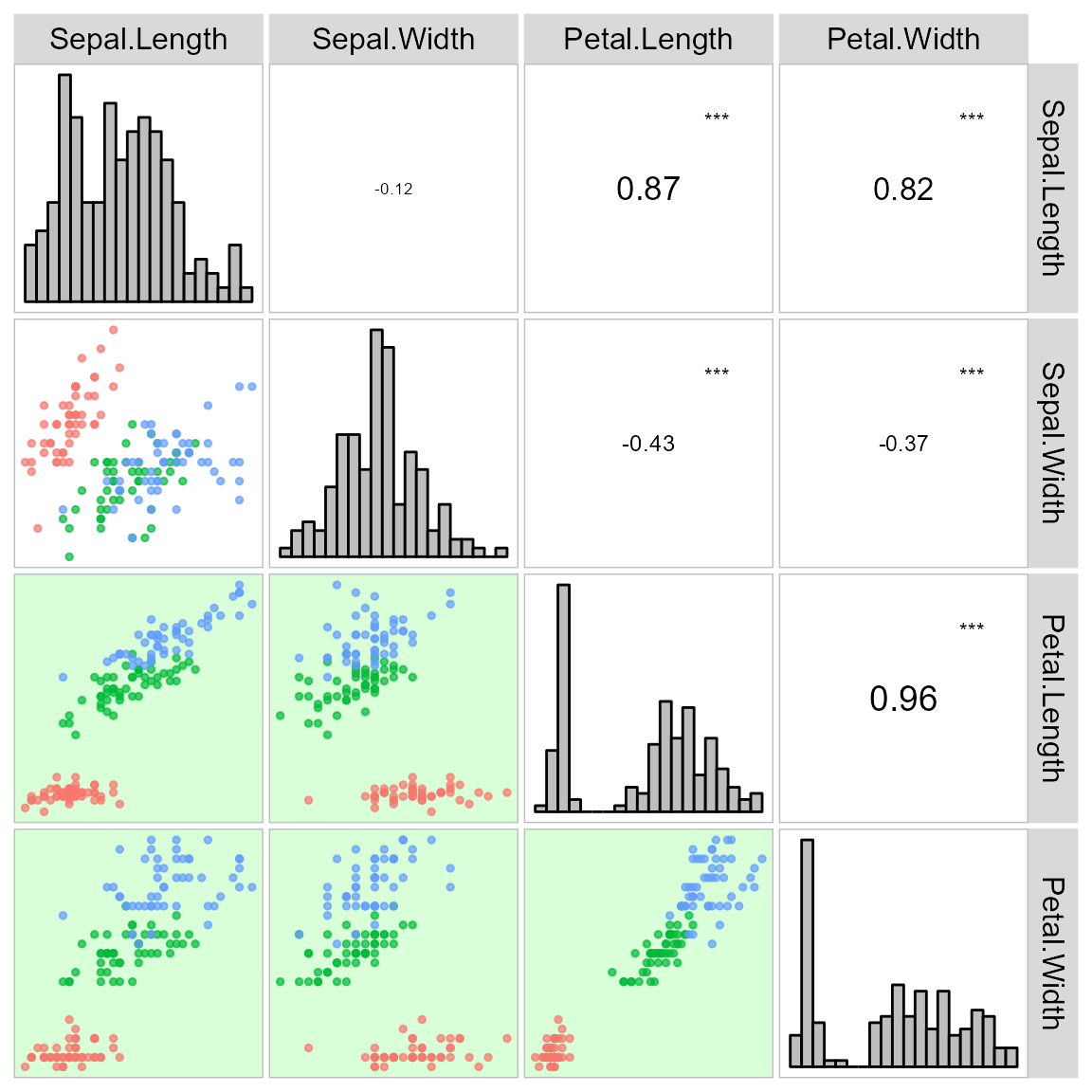
The function corr_coef() can be used to compute Pearson
product-moment correlation coefficients with p-values. A correlation
heat map can be created with the function plot()
(co)variance and correlations for designed experiments
The function covcor_design() may be used to compute
genetic, phenotypic and residual correlation/(co)variance matrices
through Analysis of Variance (ANOVA) method using randomized complete
block design (RCBD) or completely randomized design (CRD).
The phenotypic (\(r_p\)), genotypic (\(r_g\)) and residual (\(r_r\)) correlations are computed as follows:
\[ r^p_{xy} = \frac{cov^p_{xy}}{\sqrt{var^p_{x}var^p_{y}}} \\ r^g_{xy} = \frac{cov^g_{xy}}{\sqrt{var^g_{x}var^g_{y}}} \\ r^r_{xy} = \frac{cov^r_{xy}}{\sqrt{var^r_{x}var^r_{y}}} \]
Using Mean Squares (MS) from the ANOVA method, the variances (var) and covariances (cov) are computed as follows:
\[ cov^p_{xy} = [(MST_{x+y} - MST_x - MST_y)/2]/r \\ var^p_x = MST_x / r \\ var^p_y = MST_y / r \\ cov^r_{xy} = (MSR_{x+y} - MSR_x - MSR_y)/2 \\ var^r_x = MSR_x \\ var^r_y = MSR_y \\ cov^g_{xy} = [(cov^p_{xy} \times r) - cov^r_{xy}]/r \\ var^g_x = (MST_x - MSE_x)/r \\ var^g_y = (MST_x - MSE_y)/r \\ \]
where MST is the mean square for treatment, MSR is the mean square for residuals, and r is the number of replications.
The function covcor_design() returns a list with the
matrices of (co)variances and correlations. Specific matrices may be
returned using the argument type, as shown bellow.
Genetic correlations
# environment A1
data <- subset(data_ge2, ENV == "A1")
gcor <- covcor_design(data, gen = GEN, rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "gcor") %>%
as.data.frame()
print_table(gcor, rownames = TRUE)Phenotypic correlations
pcor <- covcor_design(data, gen = GEN, rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "pcor") %>%
as.data.frame()
print_table(pcor, rownames = TRUE)Residual correlations
rcor <- covcor_design(data,
gen = GEN,
rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "rcor") %>%
as.data.frame()
print_table(rcor, rownames = TRUE)Residual (co)variance matrix
In this example we will obtain the residual (co)variance for each environment.
cov <- covcor_design(data_ge2,
gen = GEN,
rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "rcov")The residual (co)variance matrix and the means (obtained using
type = "means") may be used into the function
mahala() to compute the Mahalanobis distance
res <- covcor_design(data, GEN, REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "rcov")
means <- covcor_design(data, GEN, REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
type = "means")
D2 <- mahala(.means = means, covar = res, inverted = FALSE) %>%
as.data.frame()
print_table(D2, rownames = TRUE)Nonparametric confidence interval for Pearson’s correlation
Recently, a Gaussian-independent estimator for the confidence
interval of Pearson’s correlation coefficient was proposed by T. Olivoto et al. (2018). This estimator is based on the
sample size and strength of associations and may be estimated using the
function corr_ci(). It is possible to estimate the
confidence interval by declaring the sample size (n) and the correlation
coefficient (r), or using a dataframe. The following code computes the
confidence interval and make a plot to show the results.
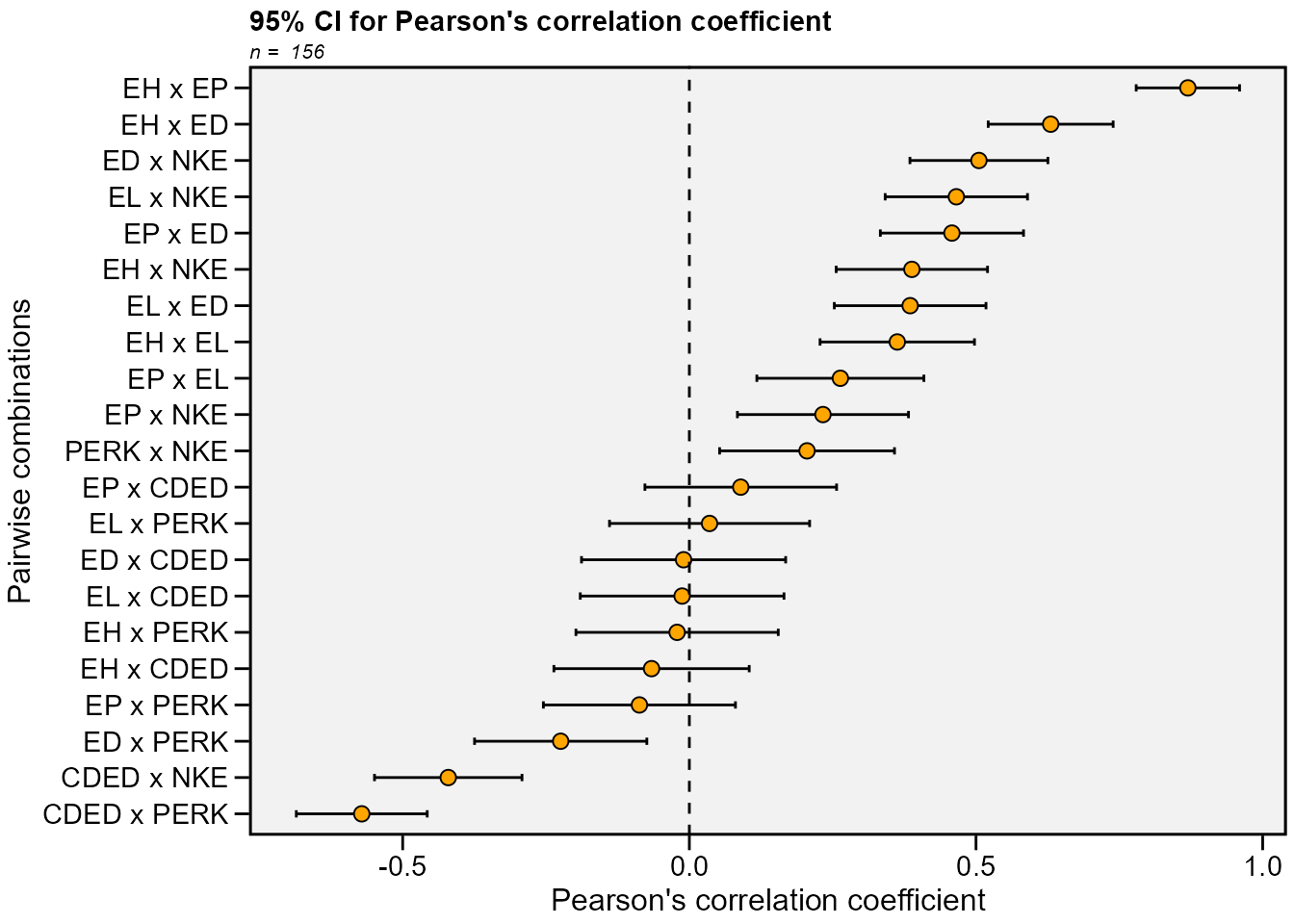
In the following examples, the confidence interval is calculated by
declaring the sample size (n) and the correlation coefficient (r). Using
the argument by = ENV the confidence interval can be
calculated within each level of the factor ENV.
# Inform n and r
corr_ci(n = 145, r = 0.34)
# -------------------------------------------------
# Nonparametric 95% half-width confidence interval
# -------------------------------------------------
# Level of significance: 5%
# Correlation coefficient: 0.34
# Sample size: 145
# Confidence interval: 0.1422
# True parameter range from: 0.1978 to 0.4822
# -------------------------------------------------
# Compute the confidence for each level of ENV
CI2 <- corr_ci(data_ge2, contains("E"), by = ENV)
# # A tibble: 21 × 7
# V1 V2 Corr n CI LL UL
# <chr> <chr> <dbl> <int> <dbl> <dbl> <dbl>
# 1 EH EP 0.839 39 0.185 0.655 1.02
# 2 EH EL 0.148 39 0.320 -0.172 0.467
# 3 EH ED -0.0385 39 0.349 -0.387 0.310
# 4 EH CDED 0.186 39 0.310 -0.125 0.496
# 5 EH PERK -0.205 39 0.306 -0.510 0.101
# 6 EH NKE -0.324 39 0.278 -0.602 -0.0458
# 7 EP EL 0.150 39 0.319 -0.169 0.469
# 8 EP ED -0.0840 39 0.336 -0.420 0.252
# 9 EP CDED 0.328 39 0.277 0.0502 0.605
# 10 EP PERK -0.221 39 0.302 -0.523 0.0801
# # … with 11 more rows
# # A tibble: 21 × 7
# V1 V2 Corr n CI LL UL
# <chr> <chr> <dbl> <int> <dbl> <dbl> <dbl>
# 1 EH EP 0.899 39 0.176 0.722 1.08
# 2 EH EL 0.498 39 0.242 0.255 0.740
# 3 EH ED 0.748 39 0.199 0.549 0.947
# 4 EH CDED 0.211 39 0.304 -0.0935 0.515
# 5 EH PERK 0.0187 39 0.354 -0.335 0.373
# 6 EH NKE 0.269 39 0.290 -0.0217 0.559
# 7 EP EL 0.365 39 0.269 0.0960 0.634
# 8 EP ED 0.600 39 0.224 0.376 0.823
# 9 EP CDED 0.249 39 0.295 -0.0463 0.544
# 10 EP PERK -0.0505 39 0.345 -0.396 0.295
# # … with 11 more rows
# # A tibble: 21 × 7
# V1 V2 Corr n CI LL UL
# <chr> <chr> <dbl> <int> <dbl> <dbl> <dbl>
# 1 EH EP 0.844 39 0.184 0.659 1.03
# 2 EH EL -0.0977 39 0.333 -0.430 0.235
# 3 EH ED 0.363 39 0.270 0.0935 0.633
# 4 EH CDED -0.282 39 0.287 -0.569 0.00544
# 5 EH PERK 0.126 39 0.325 -0.199 0.451
# 6 EH NKE 0.279 39 0.288 -0.00876 0.567
# 7 EP EL -0.194 39 0.308 -0.502 0.114
# 8 EP ED 0.200 39 0.307 -0.107 0.507
# 9 EP CDED -0.00956 39 0.357 -0.366 0.347
# 10 EP PERK 0.0365 39 0.349 -0.313 0.386
# # … with 11 more rows
# # A tibble: 21 × 7
# V1 V2 Corr n CI LL UL
# <chr> <chr> <dbl> <int> <dbl> <dbl> <dbl>
# 1 EH EP 0.781 39 0.194 0.587 0.975
# 2 EH EL 0.250 39 0.295 -0.0446 0.545
# 3 EH ED 0.404 39 0.261 0.143 0.665
# 4 EH CDED 0.143 39 0.321 -0.178 0.464
# 5 EH PERK 0.250 39 0.295 -0.0447 0.545
# 6 EH NKE 0.246 39 0.296 -0.0492 0.542
# 7 EP EL 0.241 39 0.297 -0.0559 0.538
# 8 EP ED 0.210 39 0.304 -0.0942 0.514
# 9 EP CDED 0.236 39 0.298 -0.0626 0.534
# 10 EP PERK 0.156 39 0.318 -0.162 0.473
# # … with 11 more rowsSample size planning
corr_ss(r = 0.6, CI = 0.1)
# -------------------------------------------------
# Sample size planning for correlation coefficient
# -------------------------------------------------
# Level of significance: 5%
# Correlation coefficient: 0.6
# 95% half-width CI: 0.1
# Required sample size: 194
# -------------------------------------------------Collinearity diagnostic
The following codes compute a complete collinearity diagnostic of a
correlation matrix of predictor traits. Several indicators, such as
Variance Inflation Factor, Condition Number, and Matrix Determinant are
considered (T. Olivoto et al. 2017; Olivoto T. et al.
2017) The diagnostic may be performed using: (i) correlation
matrices; (ii) dataframes, or (iii) an object of class
group_factor, which split a dataframe into subsets based on
one or more grouping factors.
Using a dataframe
cold2 <- colindiag(data_ge2)Perform the diagnostic for each level of the factor ENV
cold3 <- colindiag(data_ge2 , by = ENV)
Path analysis
Using KW as response trait and all other ones as predictors.
pcoeff <- path_coeff(data_ge2, resp = KW)
# Severe multicollinearity.
# Condition Number: 7865.84
# Consider using a correction factor with 'correction' argument.
# Consider identifying collinear traits with `non_collinear_vars()`Declaring the predictor traits
pcoeff2 <-
path_coeff(data_ge2,
resp = KW,
pred = c(PH, NKE, TKW),
verbose = FALSE)
print(pcoeff2)
# ----------------------------------------------------------------------------------------------
# Correlation matrix between the predictor traits
# ----------------------------------------------------------------------------------------------
# PH NKE TKW
# PH 1.0000 0.45838 0.56854
# NKE 0.4584 1.00000 -0.06516
# TKW 0.5685 -0.06516 1.00000
# ----------------------------------------------------------------------------------------------
# Vector of correlations between dependent and each predictor
# ----------------------------------------------------------------------------------------------
# PH NKE TKW
# KW 0.7534439 0.6810756 0.6730371
# ----------------------------------------------------------------------------------------------
# Multicollinearity diagnosis and goodness-of-fit
# ----------------------------------------------------------------------------------------------
# Condition number: 7.1689
# Determinant: 0.4284438
# R-square: 0.981
# Residual: 0.1379
# Response: KW
# Predictors: PH NKE TKW
# ----------------------------------------------------------------------------------------------
# Variance inflation factors
# ----------------------------------------------------------------------------------------------
# # A tibble: 3 × 2
# VAR VIF
# <chr> <dbl>
# 1 PH 2.324
# 2 NKE 1.580
# 3 TKW 1.844
# ----------------------------------------------------------------------------------------------
# Eigenvalues and eigenvectors
# ----------------------------------------------------------------------------------------------
# # A tibble: 3 × 4
# Eigenvalues PH NKE TKW
# <dbl> <dbl> <dbl> <dbl>
# 1 1.699 0.7222 0.4223 0.5478
# 2 1.064 -0.01921 -0.7794 0.6262
# 3 0.2370 0.6914 -0.4628 -0.5548
# ----------------------------------------------------------------------------------------------
# Variables with the largest weight in the eigenvalue of smallest magnitude
# ----------------------------------------------------------------------------------------------
# PH > TKW > NKE
# ----------------------------------------------------------------------------------------------
# Direct (diagonal) and indirect (off-diagonal) effects
# ----------------------------------------------------------------------------------------------
# PH NKE TKW linear
# PH 0.02350989 0.32834888 0.40158514 0.7534439
# NKE 0.01077646 0.71632454 -0.04602544 0.6810756
# TKW 0.01336628 -0.04667561 0.70634648 0.6730371
# ----------------------------------------------------------------------------------------------Selecting traits to be excluded from the analysis.
pcoeff2 <-
path_coeff(data_ge2,
resp = KW,
pred = c(PH, EH, NKE, TKW),
exclude = TRUE,
verbose = FALSE)Selecting a set of predictors with minimal multicollinearity
pcoeff3 <-
path_coeff(data_ge2,
resp = KW,
brutstep = TRUE,
maxvif = 5)
# --------------------------------------------------------------------------
# The algorithm has selected a set of 8 predictors with largest VIF = 3.346.
# Selected predictors: NR PERK EP CDED EL NKR TKW PH
# A forward stepwise-based selection procedure will fit 6 models.
# --------------------------------------------------------------------------
# Adjusting the model 1 with 7 predictors (16.67% concluded)
# Adjusting the model 2 with 6 predictors (33.33% concluded)
# Adjusting the model 3 with 5 predictors (50% concluded)
# Adjusting the model 4 with 4 predictors (66.67% concluded)
# Adjusting the model 5 with 3 predictors (83.33% concluded)
# Adjusting the model 6 with 2 predictors (100% concluded)
# Done!
# --------------------------------------------------------------------------
# Summary of the adjusted models
# --------------------------------------------------------------------------
# Model AIC Numpred CN Determinant R2 Residual maxVIF
# MODEL_1 1127 7 13.67 0.0841 0.933 0.259 2.59
# MODEL_2 1125 6 12.26 0.1383 0.933 0.259 2.46
# MODEL_3 1126 5 12.05 0.1989 0.932 0.261 2.31
# MODEL_4 1251 4 6.66 0.4016 0.846 0.393 1.98
# MODEL_5 1308 3 3.05 0.7438 0.774 0.475 1.34
# MODEL_6 1329 2 2.23 0.8555 0.738 0.512 1.17
# --------------------------------------------------------------------------Compute the analysis for each level of environment
pcoeff4 <-
path_coeff(data_ge2,
resp = KW,
pred = c(PH, EH, NKE, TKW),
by = ENV)
# Weak multicollinearity.
# Condition Number: 5.183
# You will probably have path coefficients close to being unbiased.
# Weak multicollinearity.
# Condition Number: 81.987
# You will probably have path coefficients close to being unbiased.
# Weak multicollinearity.
# Condition Number: 24.788
# You will probably have path coefficients close to being unbiased.
# Weak multicollinearity.
# Condition Number: 10.276
# You will probably have path coefficients close to being unbiased.Canonical correlation analysis
First of all, we will rename the plant-related traits
PH, EH EP with the suffix _PLA to show the
usability of the select helper contains().
data_cc <- rename(data_ge2,
PH_PLA = PH,
EH_PLA = EH,
EP_PLA = EP)
# Type the variable names
cc1 <- can_corr(data_cc,
FG = c(PH_PLA, EH_PLA, EP_PLA),
SG = c(EL, ED, CL, CD, CW, KW))
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between variables of first group (FG)
# ---------------------------------------------------------------------------
# PH_PLA EH_PLA EP_PLA
# PH_PLA 1.0000000 0.9318282 0.6384123
# EH_PLA 0.9318282 1.0000000 0.8695460
# EP_PLA 0.6384123 0.8695460 1.0000000
# ---------------------------------------------------------------------------
# Collinearity within first group
# ---------------------------------------------------------------------------
# The multicollinearity in the matrix should be investigated.
# CN = 977.586
# Largest VIF = 229.164618380199
# Matrix determinant: 0.0025852
# Largest correlation: PH_PLA x EH_PLA = 0.932
# Smallest correlation: PH_PLA x EP_PLA = 0.638
# Number of VIFs > 10: 3
# Number of correlations with r >= |0.8|: 2
# Variables with largest weight in the last eigenvalues:
# EH_PLA > PH_PLA > EP_PLA
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between variables of second group (SG)
# ---------------------------------------------------------------------------
# EL ED CL CD CW KW
# EL 1.0000000 0.3851451 0.2554068 0.9118653 0.4581728 0.6685601
# ED 0.3851451 1.0000000 0.6974629 0.3897128 0.7371305 0.8241426
# CL 0.2554068 0.6974629 1.0000000 0.3003636 0.7383379 0.4709310
# CD 0.9118653 0.3897128 0.3003636 1.0000000 0.4840299 0.6259806
# CW 0.4581728 0.7371305 0.7383379 0.4840299 1.0000000 0.7348622
# KW 0.6685601 0.8241426 0.4709310 0.6259806 0.7348622 1.0000000
# ---------------------------------------------------------------------------
# Collinearity within second group
# ---------------------------------------------------------------------------
# Weak multicollinearity in the matrix
# CN = 66.084
# Matrix determinant: 0.0028626
# Largest correlation: EL x CD = 0.912
# Smallest correlation: EL x CL = 0.255
# Number of VIFs > 10: 0
# Number of correlations with r >= |0.8|: 2
# Variables with largest weight in the last eigenvalues:
# KW > EL > ED > CD > CL > CW
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between FG and SG
# ---------------------------------------------------------------------------
# EL ED CL CD CW KW
# PH_PLA 0.3801960 0.6613148 0.3251648 0.3153910 0.5047388 0.7534439
# EH_PLA 0.3626537 0.6302561 0.3971935 0.2805118 0.5193136 0.7029469
# EP_PLA 0.2634237 0.4580196 0.3908239 0.1750448 0.4248098 0.4974193
# ---------------------------------------------------------------------------
# Correlation of the canonical pairs and hypothesis testing
# ---------------------------------------------------------------------------
# Var Percent Sum Corr Lambda Chisq DF p_val
# U1V1 0.630438540 78.617161 78.61716 0.7940016 0.30668 177.29224 18 0.00000
# U2V2 0.163384310 20.374406 98.99157 0.4042083 0.82985 27.97651 10 0.00182
# U3V3 0.008086721 1.008433 100.00000 0.0899262 0.99191 1.21794 4 0.87514
# ---------------------------------------------------------------------------
# Canonical coefficients of the first group
# ---------------------------------------------------------------------------
# U1 U2 U3
# PH_PLA 2.609792 5.490798 7.575090
# EH_PLA -2.559005 -7.646096 -12.812234
# EP_PLA 1.191023 2.428742 6.604968
# ---------------------------------------------------------------------------
# Canonical coefficients of the second group
# ---------------------------------------------------------------------------
# V1 V2 V3
# EL -0.01008726 -1.0481893 0.60553720
# ED 0.14629899 0.7853469 -1.30457763
# CL -0.09112023 -1.2989864 -0.07497186
# CD -0.29105227 1.1513083 -1.50589651
# CW -0.12527616 -0.0361706 0.21180796
# KW 1.16041981 -0.1022916 1.34278026
# ---------------------------------------------------------------------------
# Canonical loads of the first group
# ---------------------------------------------------------------------------
# U1 U2 U3
# PH_PLA 0.9856022 -0.08351129 -0.1470178
# EH_PLA 0.9085216 -0.41771278 -0.0102277
# EP_PLA 0.6319736 -0.71449671 0.3001730
# ---------------------------------------------------------------------------
# Canonical loads of the second group
# ---------------------------------------------------------------------------
# V1 V2 V3
# EL 0.4759982 -0.11260907 -0.2944636
# ED 0.8294407 -0.18663860 -0.4477426
# CL 0.3749015 -0.74801793 -0.4937819
# CD 0.3951578 0.02985218 -0.5415818
# CW 0.6225367 -0.41451273 -0.2698904
# KW 0.9570820 -0.07344796 -0.1498587
# Use select helpers
cc2 <- can_corr(data_cc,
FG = contains("_PLA"),
SG = c(EL, ED, CL, CD, CW, KW))
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between variables of first group (FG)
# ---------------------------------------------------------------------------
# PH_PLA EH_PLA EP_PLA
# PH_PLA 1.0000000 0.9318282 0.6384123
# EH_PLA 0.9318282 1.0000000 0.8695460
# EP_PLA 0.6384123 0.8695460 1.0000000
# ---------------------------------------------------------------------------
# Collinearity within first group
# ---------------------------------------------------------------------------
# The multicollinearity in the matrix should be investigated.
# CN = 977.586
# Largest VIF = 229.164618380199
# Matrix determinant: 0.0025852
# Largest correlation: PH_PLA x EH_PLA = 0.932
# Smallest correlation: PH_PLA x EP_PLA = 0.638
# Number of VIFs > 10: 3
# Number of correlations with r >= |0.8|: 2
# Variables with largest weight in the last eigenvalues:
# EH_PLA > PH_PLA > EP_PLA
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between variables of second group (SG)
# ---------------------------------------------------------------------------
# EL ED CL CD CW KW
# EL 1.0000000 0.3851451 0.2554068 0.9118653 0.4581728 0.6685601
# ED 0.3851451 1.0000000 0.6974629 0.3897128 0.7371305 0.8241426
# CL 0.2554068 0.6974629 1.0000000 0.3003636 0.7383379 0.4709310
# CD 0.9118653 0.3897128 0.3003636 1.0000000 0.4840299 0.6259806
# CW 0.4581728 0.7371305 0.7383379 0.4840299 1.0000000 0.7348622
# KW 0.6685601 0.8241426 0.4709310 0.6259806 0.7348622 1.0000000
# ---------------------------------------------------------------------------
# Collinearity within second group
# ---------------------------------------------------------------------------
# Weak multicollinearity in the matrix
# CN = 66.084
# Matrix determinant: 0.0028626
# Largest correlation: EL x CD = 0.912
# Smallest correlation: EL x CL = 0.255
# Number of VIFs > 10: 0
# Number of correlations with r >= |0.8|: 2
# Variables with largest weight in the last eigenvalues:
# KW > EL > ED > CD > CL > CW
# ---------------------------------------------------------------------------
# Matrix (correlation/covariance) between FG and SG
# ---------------------------------------------------------------------------
# EL ED CL CD CW KW
# PH_PLA 0.3801960 0.6613148 0.3251648 0.3153910 0.5047388 0.7534439
# EH_PLA 0.3626537 0.6302561 0.3971935 0.2805118 0.5193136 0.7029469
# EP_PLA 0.2634237 0.4580196 0.3908239 0.1750448 0.4248098 0.4974193
# ---------------------------------------------------------------------------
# Correlation of the canonical pairs and hypothesis testing
# ---------------------------------------------------------------------------
# Var Percent Sum Corr Lambda Chisq DF p_val
# U1V1 0.630438540 78.617161 78.61716 0.7940016 0.30668 177.29224 18 0.00000
# U2V2 0.163384310 20.374406 98.99157 0.4042083 0.82985 27.97651 10 0.00182
# U3V3 0.008086721 1.008433 100.00000 0.0899262 0.99191 1.21794 4 0.87514
# ---------------------------------------------------------------------------
# Canonical coefficients of the first group
# ---------------------------------------------------------------------------
# U1 U2 U3
# PH_PLA 2.609792 5.490798 7.575090
# EH_PLA -2.559005 -7.646096 -12.812234
# EP_PLA 1.191023 2.428742 6.604968
# ---------------------------------------------------------------------------
# Canonical coefficients of the second group
# ---------------------------------------------------------------------------
# V1 V2 V3
# EL -0.01008726 -1.0481893 0.60553720
# ED 0.14629899 0.7853469 -1.30457763
# CL -0.09112023 -1.2989864 -0.07497186
# CD -0.29105227 1.1513083 -1.50589651
# CW -0.12527616 -0.0361706 0.21180796
# KW 1.16041981 -0.1022916 1.34278026
# ---------------------------------------------------------------------------
# Canonical loads of the first group
# ---------------------------------------------------------------------------
# U1 U2 U3
# PH_PLA 0.9856022 -0.08351129 -0.1470178
# EH_PLA 0.9085216 -0.41771278 -0.0102277
# EP_PLA 0.6319736 -0.71449671 0.3001730
# ---------------------------------------------------------------------------
# Canonical loads of the second group
# ---------------------------------------------------------------------------
# V1 V2 V3
# EL 0.4759982 -0.11260907 -0.2944636
# ED 0.8294407 -0.18663860 -0.4477426
# CL 0.3749015 -0.74801793 -0.4937819
# CD 0.3951578 0.02985218 -0.5415818
# CW 0.6225367 -0.41451273 -0.2698904
# KW 0.9570820 -0.07344796 -0.1498587Clustering analysis
Using function clustering()
All rows and all numeric variables from data
d1 <- clustering(data_ge2)Based on the mean for each genotype
mean_gen <-
data_ge2 %>%
mean_by(GEN) %>%
column_to_rownames("GEN")
d2 <- clustering(mean_gen)The S3 generic function plot() may be used to plot the dendrogram
generated by the function clustering(). A dashed line is
draw at the cutpoint suggested according to Mojena (1977).
plot(d2)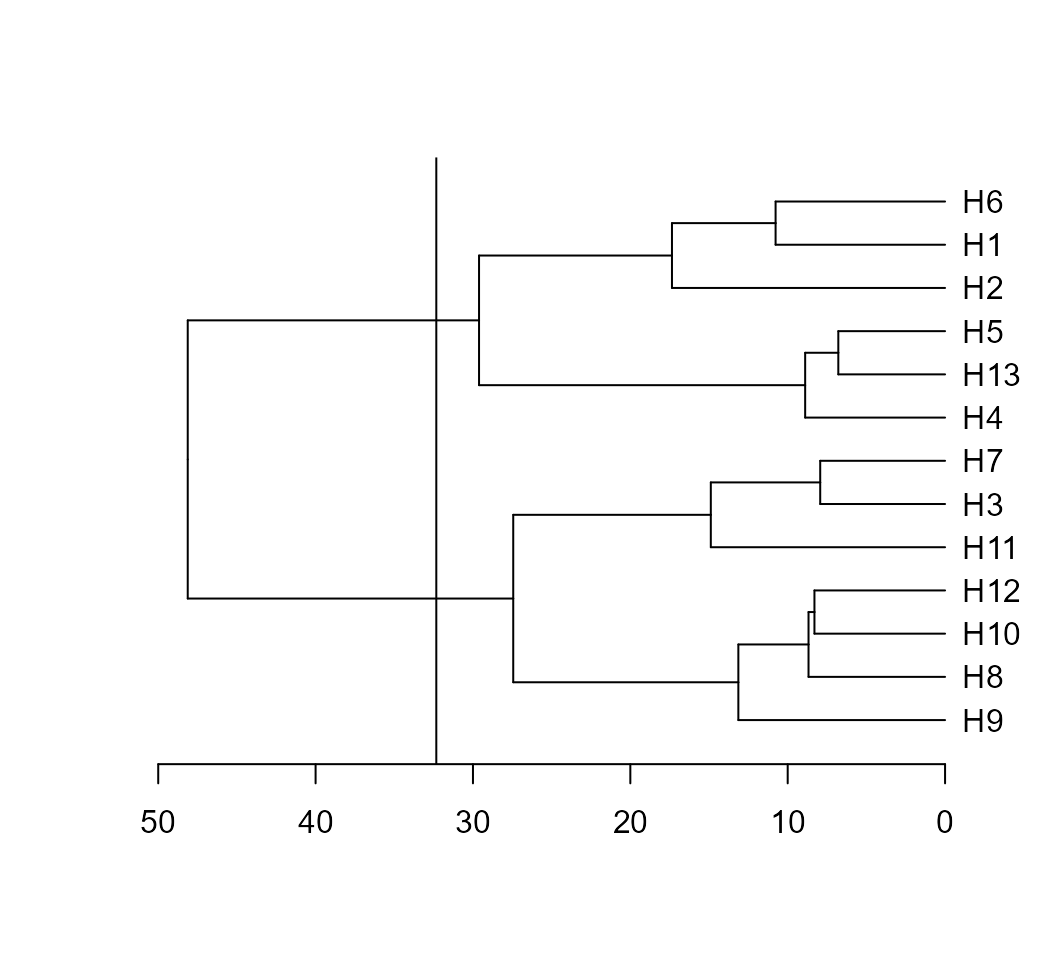 According to the suggested cutpoint, two clusters are formed. The number
of clusters may also be found using intensive computation. I suggest the
package pcvlust, an R
package for assessing the uncertainty in hierarchical cluster analysis.
The implementation may be see below.
According to the suggested cutpoint, two clusters are formed. The number
of clusters may also be found using intensive computation. I suggest the
package pcvlust, an R
package for assessing the uncertainty in hierarchical cluster analysis.
The implementation may be see below.
library(pvclust)
pv_clust <- pvclust(t(d2$data), nboot = 100, method.dist = "euclidean")
# Bootstrap (r = 0.47)... Done.
# Bootstrap (r = 0.6)... Done.
# Bootstrap (r = 0.67)... Done.
# Bootstrap (r = 0.8)... Done.
# Bootstrap (r = 0.87)... Done.
# Bootstrap (r = 1.0)... Done.
# Bootstrap (r = 1.07)... Done.
# Bootstrap (r = 1.2)... Done.
# Bootstrap (r = 1.27)... Done.
# Bootstrap (r = 1.4)... Done.
plot(pv_clust, hang = -1, cex = 0.5)
pvrect(pv_clust, alpha = 0.95)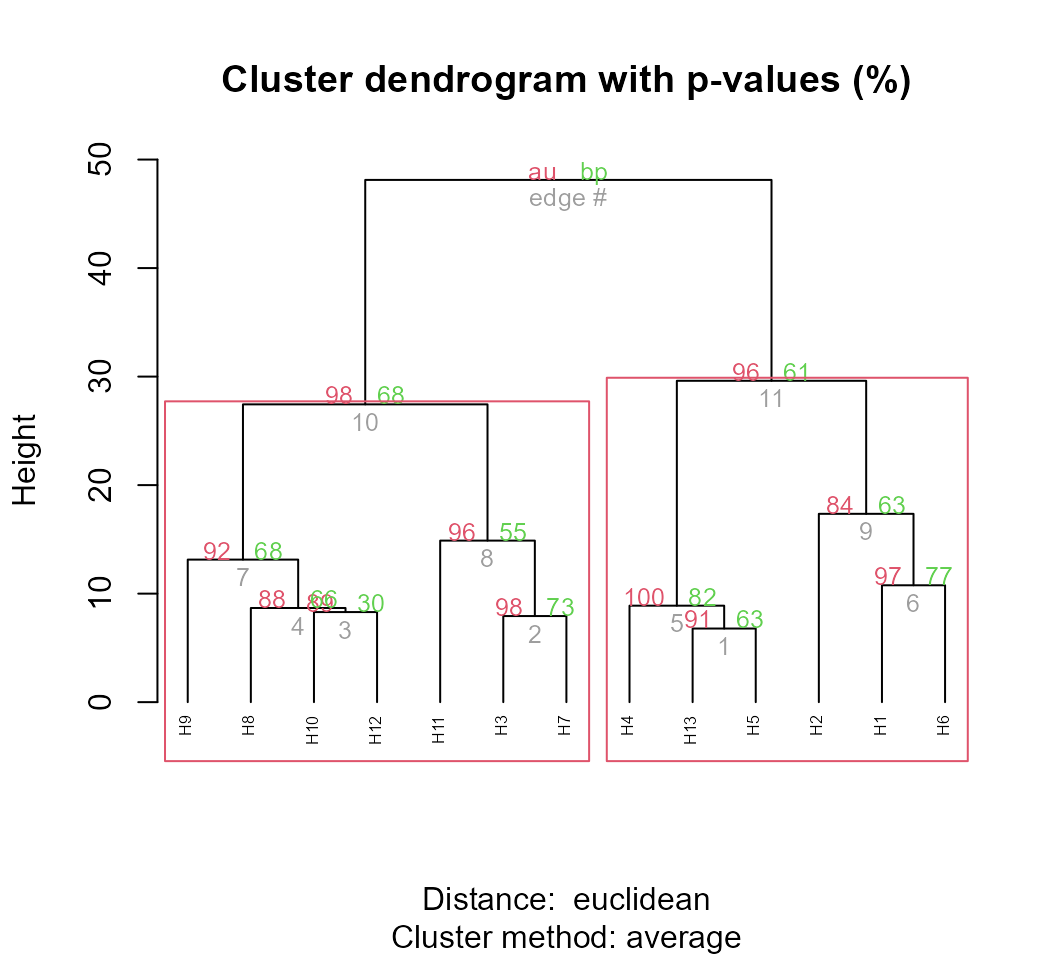
Indicating the variables to compute the distances
It is possible to indicate the variables from the data_ge2 to compute the distances. To do that is easy. You should only provide a comma-separated list of unquoted variable names after the .data argument. For example, to compute the distances between the genotypes based on the variables NKR, TKW, and NKE, the following arguments should be used.
d3 <- clustering(mean_gen, NKR, TKW, NKE)Select variables for compute the distances
When selvar = TRUE is used, an algorithm for variable
selection is implemented. See ?clustering for more
details.
d4 <- clustering(mean_gen, selvar = TRUE)
# EH excluded in this step |=== | 7%
EP excluded in this step |======= | 14%
CDED excluded in this step |========== | 21%
PH excluded in this step |============== | 29%
CL excluded in this step |================= | 36%
NR excluded in this step |===================== | 43%
PERK excluded in this step |======================= | 50%
EL excluded in this step |=========================== | 57%
CD excluded in this step |=============================== | 64%
ED excluded in this step |================================== | 71%
KW excluded in this step |====================================== | 79%
CW excluded in this step |========================================= | 86%
NKR excluded in this step |============================================ | 93%
TKW excluded in this step |===============================================| 100%
# --------------------------------------------------------------------------
#
# Summary of the adjusted models
# --------------------------------------------------------------------------
# Model excluded cophenetic remaining cormantel pvmantel
# Model 1 - 0.8656190 15 1.0000000 0.000999001
# Model 2 EH 0.8656191 14 1.0000000 0.000999001
# Model 3 EP 0.8656191 13 1.0000000 0.000999001
# Model 4 CDED 0.8656191 12 1.0000000 0.000999001
# Model 5 PH 0.8656189 11 1.0000000 0.000999001
# Model 6 CL 0.8655939 10 0.9999996 0.000999001
# Model 7 NR 0.8656719 9 0.9999982 0.000999001
# Model 8 PERK 0.8657259 8 0.9999977 0.000999001
# Model 9 EL 0.8657904 7 0.9999972 0.000999001
# Model 10 CD 0.8658997 6 0.9999964 0.000999001
# Model 11 ED 0.8658274 5 0.9999931 0.000999001
# Model 12 KW 0.8643556 4 0.9929266 0.000999001
# Model 13 CW 0.8640355 3 0.9927593 0.000999001
# Model 14 NKR 0.8648384 2 0.9925396 0.000999001
# --------------------------------------------------------------------------
# Suggested variables to be used in the analysis
# --------------------------------------------------------------------------
# The clustering was calculated with the Model 10
# The variables included in this model were...
# ED CW KW NKR TKW NKE
# --------------------------------------------------------------------------The distances were computed using the variables ED, CW, KW, NKR, TKW, and NKE. By using these variables the highest cophenetic correlation coefficient (0.8658) was observed. The Mantel’s correlation estimated with the distance matrix of Model 10 (selected variables) with the original distance matrix (estimated with all variables) was near to 1, suggesting that the deletion of the variables to compute the distance don’t affect significantly the computation of the distances.
Extending the dendrogram Functionality
The package dendextend offers a set of functions for extending ‘dendrogram’ objects in R. A simple example is given bellow.
library(dendextend)
d4$hc %>%
color_labels(k = 2, col = c("red", "blue")) %>%
branches_color(k = 2, col = c("red", "blue")) %>%
highlight_branches_lwd() %>%
plot(horiz = TRUE, xlab = "Euclidean distance")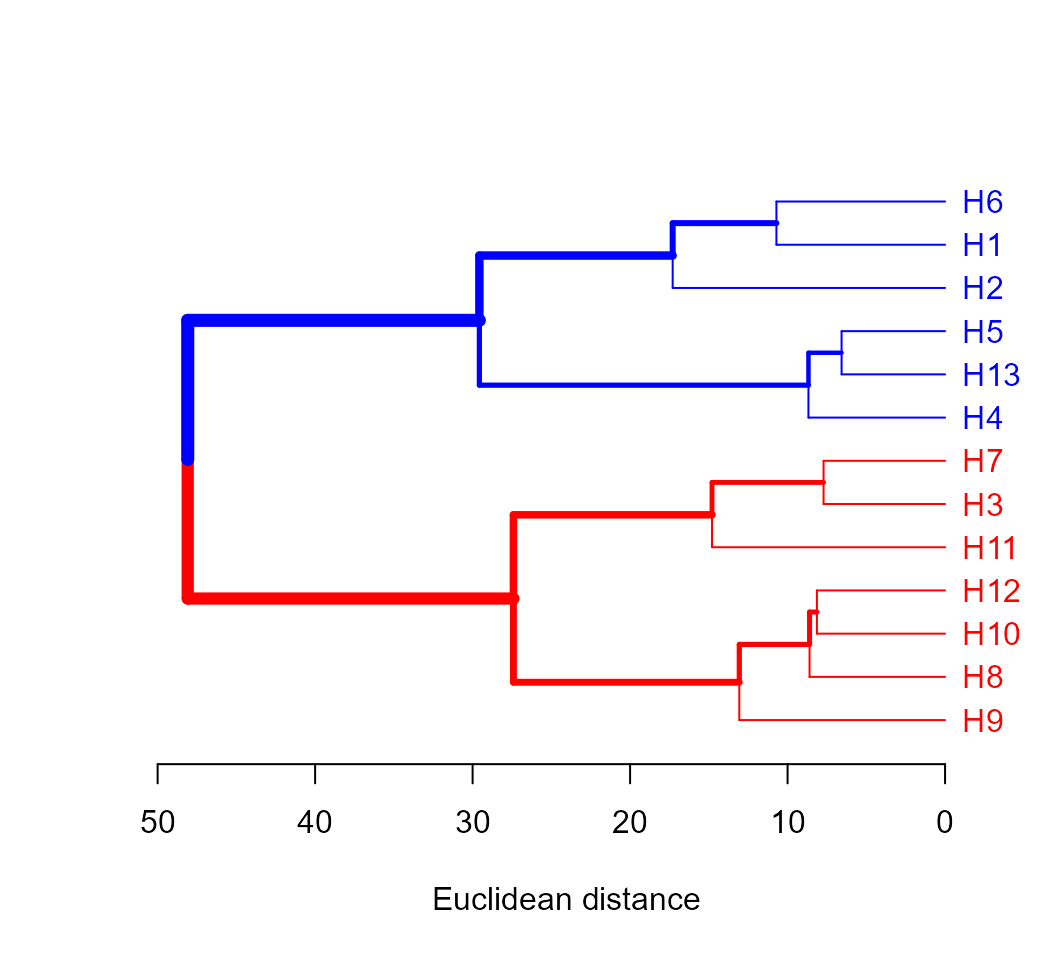
Compute the distances for each environment
- All rows of each environment and all numeric variables used
d6 <- clustering(data_ge2,
by = ENV,
nclust = 2)Check the correlation between distance matrices
The function pairs_mantel() may be used to check the
relationships between the distance matrices when the clustering is
performed for each level of a grouping factor. In this example, we have
four distance matrices corresponding to four environments.
pairs_mantel(d6, names = c("A1", "A2", "A3", "A4"))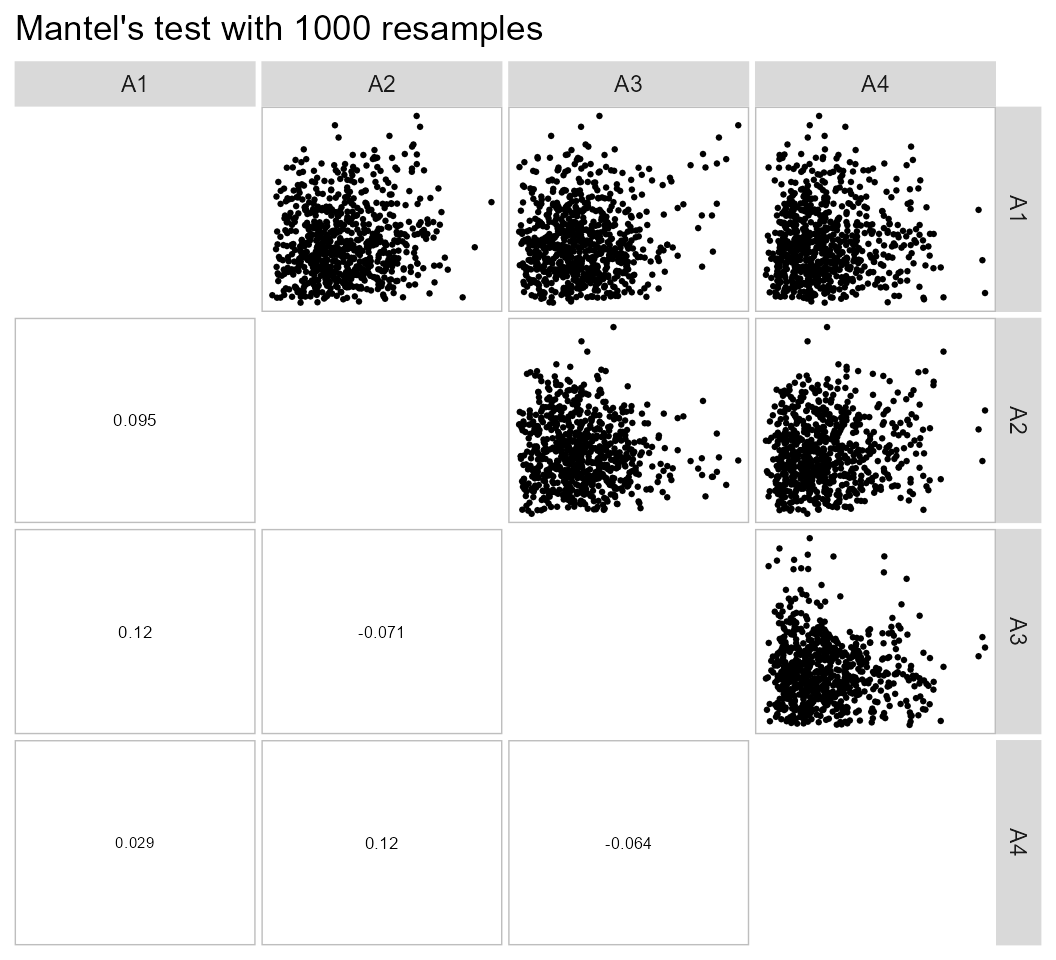
The low values of correlation between the distance matrices suggest that the genotype clustering should vary significantly among environments.
Mahalanobis distance
Based on designed experiments
# Environment E1
data_E1 <- subset(data_ge2, ENV == "A1")
D2_des <- mahala_design(data_E1,
gen = GEN,
rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW)) %>%
as.data.frame()
print_table(D2_des, rownames = TRUE)Compute one distance for each environment
To compute the Mahalanobis distance for each environment (or any
grouping variable) we can use the argument by. Note that
pairs_mantel() is used to compare compute the Mantel’s test
for each combination of distance matrices. Let’s do it.
data_ge2 %>%
mahala_design(gen = GEN,
rep = REP,
resp = c(PH, EH, NKE, TKW, CL, CD, CW, KW),
by = ENV)
# # A tibble: 52 × 14
# ENV H1 H10 H11 H12 H13 H2 H3 H4 H5 H6 H7 H8
# <fct> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
# 1 A1 0 24.3 21.0 21.5 19.0 29.0 20.2 10.7 19.1 16.7 26.9 33.9
# 2 A1 24.3 0 4.64 7.63 28.4 20.4 12.4 24.5 27.8 23.6 18.0 12.9
# 3 A1 21.0 4.64 0 7.05 35.4 15.9 9.21 17.2 18.7 27.1 15.9 16.5
# 4 A1 21.5 7.63 7.05 0 18.2 12.5 6.66 13.4 15.4 20.6 6.11 6.69
# 5 A1 19.0 28.4 35.4 18.2 0 28.5 19.1 15.4 22.0 7.33 17.1 17.3
# 6 A1 29.0 20.4 15.9 12.5 28.5 0 7.01 19.9 25.6 33.2 24.9 23.8
# 7 A1 20.2 12.4 9.21 6.66 19.1 7.01 0 6.99 12.1 20.0 11.0 15.2
# 8 A1 10.7 24.5 17.2 13.4 15.4 19.9 6.99 0 5.49 13.8 11.3 22.3
# 9 A1 19.1 27.8 18.7 15.4 22.0 25.6 12.1 5.49 0 14.0 6.40 16.3
# 10 A1 16.7 23.6 27.1 20.6 7.33 33.2 20.0 13.8 14.0 0 15.0 14.1
# # … with 42 more rows, and 1 more variable: H9 <dbl>If I have the matrices of means and covariances
Lets suppose we want compute the Mahalanobis’ distance for each
pairwise genotype comparison based on cob-related traits. Note that the
function select(contains("C")) is used to select the
cob-relate traits, after computing the mean for each genotype.
The next step is to compute the variance-covariance matrix for the
means. The first approach combines R base functions with some functions
from metan package to compute the covariance matrix. Of
course, the simplest way is by using cov().
# Compute the covariance matrix (by hand)
cov_mat <- matrix(0, 4, 4)
dev_scores <- sweep(means, 2, colMeans(means), FUN = "-")
comb_v <- comb_vars(dev_scores, FUN = "*")
cov_mat[lower.tri(cov_mat, diag = F)] <- colSums(comb_v/(nrow(means) - 1))
rownames(cov_mat) <- colnames(cov_mat) <- colnames(means)
cov_mat <- make_sym(cov_mat, diag = diag(var(means)))
# Compute the covariance using cov()
covmat2 <- cov(means)
# Check if the two matrices are equal
all.equal(cov_mat, covmat2)
# [1] TRUEAfter computing the means and covariance matrices we are able to
compute the Mahalanobis distance using the function
mahala().
D2 <- mahala(means, covar = cov_mat)
# Dendrogram
D2 %>% as.dist() %>% hclust() %>% as.dendrogram() %>% plot()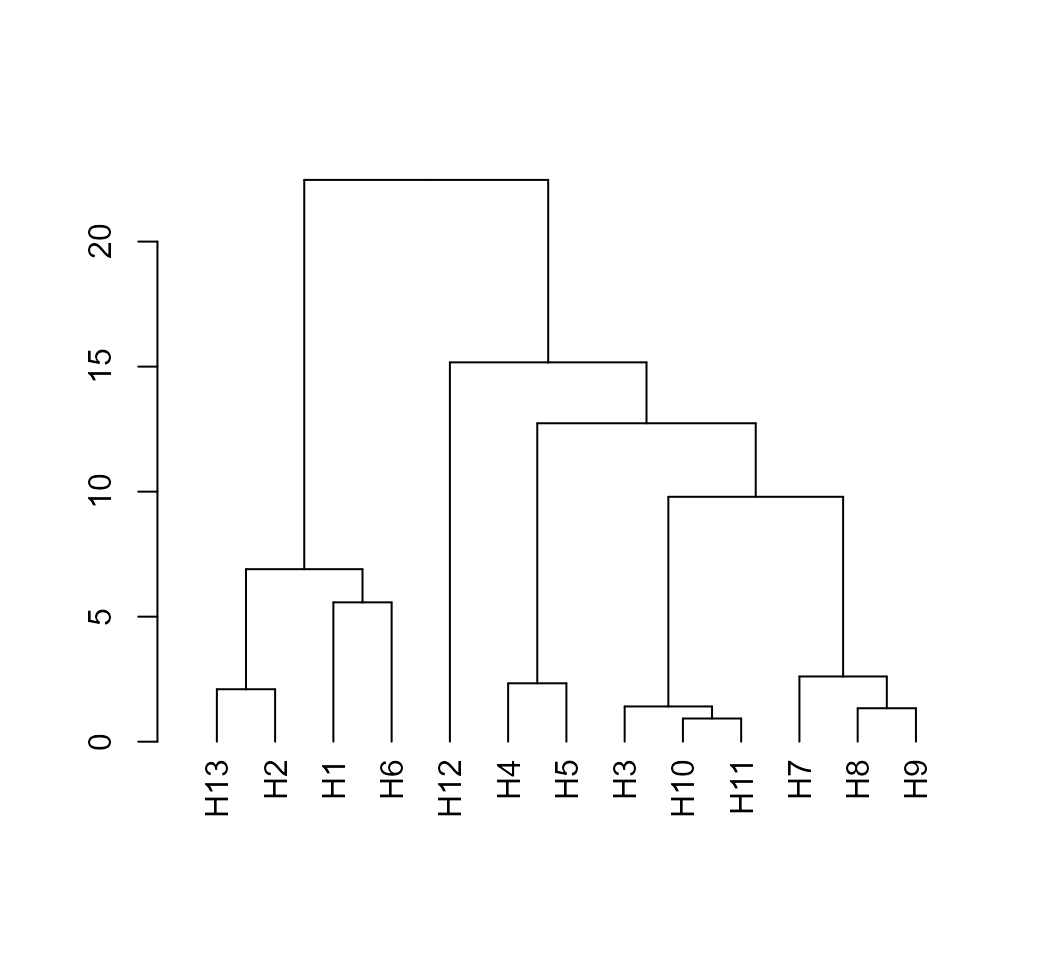
Rendering engine
This vignette was built with pkgdown. All tables were produced
with the package DT using the
following function.
library(DT) # Used to make the tables
# Function to make HTML tables
print_table <- function(table, rownames = FALSE, digits = 3, ...){
datatable(table, rownames = rownames, extensions = 'Buttons',
options = list(scrollX = TRUE,
dom = '<<t>Bp>',
buttons = c('copy', 'excel', 'pdf', 'print')), ...) %>%
formatSignif(columns = c(as.numeric(which(sapply(table, class) == "numeric"))), digits = digits)}#References